| View previous topic :: View next topic |
| Author |
Message |
bugrock

Joined: 24 Nov 2008
Posts: 137
Location: Michigan


|
 Posted: May 27, 2010 21:09 Post subject: Acid 'Dipping' of Calcite/ Acid treatment to liberate minerals in Calcite Posted: May 27, 2010 21:09 Post subject: Acid 'Dipping' of Calcite/ Acid treatment to liberate minerals in Calcite |
|
|
Hello all,
Interested in comments regarding acid treatment of calcite. One form is called acid "dipping". My understanding is this is used to make the surface of calcite look smoother and takes out small nicks and dings but if you look with magnification you can tell that the process rounds out crystal edges and points and makes the piece look artificially 'smooth'. Very interested to know how you can spot this process if you are examining a piece, such as at a mineral show [what I have mentioned may be the clues; are there others?].
A related subject is more prolonged acid treatment to reveal enclosed minerals such as copper xls (used in the Keweenaw of Michigan with great frequency for mine-pile pieces, often to reveal copper xls). But I think there are other regions where this process is used. For instance South African hematite xls [and other minerals]. They often occur with calcite. Several times I have asked those who sell SA minerals this question but details are not provided. I suspect such treatment is used regardless. Calcite is just too easy to remove with weak acids.
Actually, unless a piece is subjected to dyes or dishonestly treated by methods to make one mineral simulate another I have little problem. But just wish that preparation methods where freely described and admitted [and even included on the specimen labels ! ].
The recent Rogerley Mine article ( https://www.mineral-forum.com/message-board/viewtopic.php?t=1068 ) is very informative and as I have never collected in a mine that article was a revelation. I can actually imagine a shelf in a mineral cabinet, raw pieces on the left half, prepared specimens on the right; similar contrast as presented in the
Rogerley mine article. A focus of conversation.
In the mineral collecting community there is considerable attention to provenance and to locality attribution. Why is there such limited attention to preparation? Is it one of those dark subjects we wish to sweep under the rug? It's time for more honesty.
To all,
Take care
|
|
| Back to top |
|
 |
Peter Megaw
Site Admin

Joined: 13 Jan 2007
Posts: 963
Location: Tucson, Arizona



|
 Posted: May 27, 2010 21:46 Post subject: Re: Acid 'Dipping' of Calcite/ Acid treatment to liberate minerals in Calcite Posted: May 27, 2010 21:46 Post subject: Re: Acid 'Dipping' of Calcite/ Acid treatment to liberate minerals in Calcite |
|
|
First let me direct you to the "Cleaning and Preparing" heading on the home page...you will see that manifold aspects of cleaning, trimming, repairing and disclosure of same is a time-honored topic here on FMF....no carpet sweeping under around here! Jesse did a huge service in demonstrating the realities of making specimens marketable/presentable and if it wouldn't cut into his sales too much, I'd invite him to put in a case at Tucson showing the stages between before and after.
As to recognizing acid dipped calcite...you've got the general idea already, but if you hold it to the light at an angle you can see a roundness to the angles and tiny growth faces that should all be nice and sharp...very obvious with a handlens. As long as you handle with care, the dealer should not be surprised to see you do this. For orientation, check out a chunk of the blue, green or orange massive acid dipped stuff they sell in gift shops and airports almost everywhere and you'll see an extreme example...the more localized application looks the same in deteail. Since calcite is so soft and has perfect cleavage the first place to look is crystal tips and exposed edges where damage can easily occur. The flip side of making things shiny is to acid rough cleavage surfaces so they're less reflective and obvious.
As to removing calcite from anything, it is a very common practise...in many cases calcite is just considered good protection for delicate crystals or surfaces and it is expected to be removed from the get-go. Sinkankis "Gemstone and Mineral Databook" contains a wealth of information about the best means for removing calcite depending on the underlying mineral, preventing tarnish, and importantly, safe handling of the acids used. Collateral damage is very common, so make sure you use the right chemical for the job. Many commercial preparers have proprietary mixtures that are very good for removing specific minerals from specific localities...they guard these recipes like Coca Cola guards theirs.
_________________
Siempre Adelante! |
|
| Back to top |
|
 |
bugrock

Joined: 24 Nov 2008
Posts: 137
Location: Michigan


|
 Posted: May 27, 2010 23:05 Post subject: Re: Acid 'Dipping' of Calcite/ Acid treatment to liberate minerals in Calcite Posted: May 27, 2010 23:05 Post subject: Re: Acid 'Dipping' of Calcite/ Acid treatment to liberate minerals in Calcite |
|
|
Peter (All),
I am most appreciative of your comments and I wish more would come 'out of the/their closet' on this
subject.
More comments from all, please.
Will note one other process that occurs in the Keweenaw region for 'mine pile' copper.
Only told of this process second and third hand but pretty certain it occurs and with some
frequency.
First, take off the calcite with weak acid (sulfamic favored in our region).
Second, once the calcite is off and the piece looks copper penny shiny you don't want greenish secondary
minerals to coat the surface and you do want to impart the 'antique' patina. So boil the piece in hydrogen
peroxide and you get that dark oxidized patina. OK, further preserve/enhance that 'dark patina' with a coat of
WD-40 and thus further prevent any inconvenient green-blue coatings. But this last step leaves an odor and
if the piece is displayed on any white surface (cotton, cardboard, etc) an oily stain. Those aware of the process can
search for these features. Pick it up and see if it has a hydrocarbon scent.
This unroofs another possible topic but more specific for the Keweenaw coppers perhaps? How do you
distinguish a mine pile and treated copper specimen from one that historically came from a mine when it
was working and had a "real antique patina"? I have seen a few copper specimens from
our district that have both a reddish and very dark (nearly black) patina on the same specimen. I suspect
these are the real beast. How could you manufacture a two toned patina? If anyone knows of a method
let me know, perhaps it has been tried and accomplished? if so I have run out of physical methods to verify
a 'real" copper and will just concentrate on the xl structure [or can that also be manipulated?].
Slowly I learn more and more about minerals and their preparation.
Please join the discussion!
George
|
|
| Back to top |
|
 |
alfredo
Site Admin

Joined: 30 Jan 2008
Posts: 979



|
 Posted: May 28, 2010 00:34 Post subject: Re: Acid 'Dipping' of Calcite/ Acid treatment to liberate minerals in Calcite Posted: May 28, 2010 00:34 Post subject: Re: Acid 'Dipping' of Calcite/ Acid treatment to liberate minerals in Calcite |
|
|
| Two-toned patinas are easy. I accidentally got a 2-toned patina once on a big pyrite specimen that I'd put in concentrated Na(OH) to get rid of some dusty sulphosalts. A patina appeared on the pyrite that was differently colored from the one under the previous sulphosalts; patchy. Got the red/black patina on copper too, on a big piece that was half sticking out of its bath. Things to be avoided, but easily accomplished if a person really wanted to, and if the specimen would acquire much added value. I doubt anyone would bother for a $10 rock, so any weird patinas on a cheap rock would be either natural or accidental, but on a $500 rock... the temptation would be there to "improve" on Nature, and very difficult to prove.
|
|
| Back to top |
|
 |
Matt_Zukowski
Site Admin
Joined: 10 Apr 2009
Posts: 707
Location: Alaska



|
 Posted: May 28, 2010 00:57 Post subject: Re: Acid 'Dipping' of Calcite/ Acid treatment to liberate minerals in Calcite Posted: May 28, 2010 00:57 Post subject: Re: Acid 'Dipping' of Calcite/ Acid treatment to liberate minerals in Calcite |
|
|
I think it is always a good idea to smell a specimen before purchase. I have detected hydrocarbon odors on many calcites and fluorites - and then wanted to go wash my hands.
I looked at a nice rhodo from SA that had some damaged areas and thus needed trimming. I asked the dealer what they would do to spruce it up for me if i bought it. They said not only would they trim it but that would put some oil on some of the terminations because they were damaged (which i found hard to see). I doubt this oil treatment would have been detectable by nose. This dealer was good to display their specimen as it was without oil, so I don't have any problem with them - they would oil it only if I bought and requested the treatment. But it seems likely that others have not hesitated to add some oil to some less than perfect xtal tips, and then not tell a potential buyer.
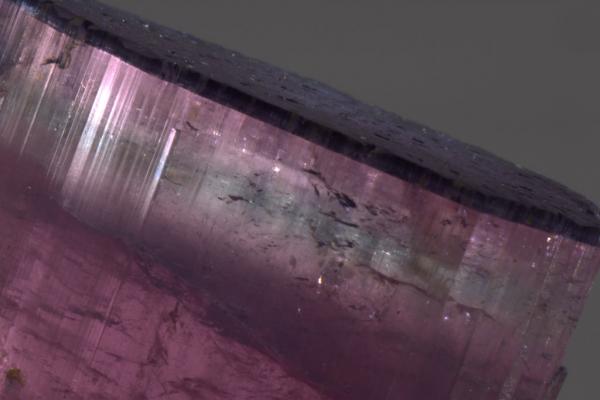
I bought a tourmaline at Tucson that clearly had issues, but it was very cool (blue/purple cap overlying a very gemmy colorless zone) and the price acceptable. The obvious issue was a repair that was covered up with sand, and there were a bunch of minor xtals around the base of the piece that could have been real but also had the same color sand so could have been glued to the piece. I bought the piece in part as an experiment to see how the mineral restoration process worked. I took a bunch of "before" pictures (see attached) and sent it to the restorer. He has since soaked it in organic solvents and all of the glue and sand fell away and also it turned out that all the small xtals were glued around the base. When i get the piece back, I will take "after" pictures and post them here.
| Description: |
|
| Viewed: |
18551 Time(s) |

|
| Description: |
|
| Viewed: |
18542 Time(s) |

|
| Description: |
|
| Viewed: |
18559 Time(s) |
|
| Description: |
|
| Viewed: |
18540 Time(s) |

|
|
|
| Back to top |
|
 |
alfredo
Site Admin

Joined: 30 Jan 2008
Posts: 979



|
 Posted: May 28, 2010 01:38 Post subject: Re: Acid 'Dipping' of Calcite/ Acid treatment to liberate minerals in Calcite Posted: May 28, 2010 01:38 Post subject: Re: Acid 'Dipping' of Calcite/ Acid treatment to liberate minerals in Calcite |
|
|
On the topic of hydrocarbon smells... Excalibur Mineral Co. once had some fluorites from the Midwest of the USA (Illinois?) that reeked of petroleum, naturally, and even had moveable bubbles of oil included inside them. I prefer igneous and metamorphic rocks myself, but those of you who collect from sedimentary rocks need to be aware that hydrocarbon smells can be natural. Watch out also for rocks with sawn bases - There can be lingering odors from the oil used on the saw.
(And the way things are going in the Gulf, those nice chalcedony corals from Florida beaches will soon have a "natural" hydrocarbon smell.)
|
|
| Back to top |
|
 |
jorge santos garcia
Joined: 06 Jan 2008
Posts: 34
Location: Évora



|
 Posted: May 28, 2010 05:47 Post subject: Re: Acid 'Dipping' of Calcite/ Acid treatment to liberate minerals in Calcite Posted: May 28, 2010 05:47 Post subject: Re: Acid 'Dipping' of Calcite/ Acid treatment to liberate minerals in Calcite |
|
|
it's not difficult to see if a calcite was acid treated. It would have a greasy look instead of the usual glassy lustre. If you handle it, yuo will feel also a greasy and smoothed touch.
At naked eye you'll see that the corners and edges are rounded and the surface marks and lines were erased.
When the acid dip is made in small nicks and dings to disguise it's white shine, you can, with a 10x loupe, easily see a mark with different shine around the nick. This treatment can be completed with a drop of oil or liquid silicone.
Jorge
|
|
| Back to top |
|
 |
Peter Megaw
Site Admin

Joined: 13 Jan 2007
Posts: 963
Location: Tucson, Arizona



|
 Posted: May 28, 2010 08:43 Post subject: Re: Acid 'Dipping' of Calcite/ Acid treatment to liberate minerals in Calcite Posted: May 28, 2010 08:43 Post subject: Re: Acid 'Dipping' of Calcite/ Acid treatment to liberate minerals in Calcite |
|
|
The fluorites from Muzquiz, Coahuila Mexico contain abundant natural petroleum bearing inclusions...a slight scratch liberates the smell immediately...and trimming them can be a heady experience like sniffing gasoline!
Many restorers use silicon oil which is colorless and odorless...it comes in a liquid and a spray and can be hard to detect.
_________________
Siempre Adelante! |
|
| Back to top |
|
 |
|


