| View previous topic :: View next topic |
| Author |
Message |
Parvin
Joined: 27 Aug 2014
Posts: 22
Location: Georgia


|
 Posted: Nov 10, 2014 13:01 Post subject: Why does Calcite fluoresce blue?? Posted: Nov 10, 2014 13:01 Post subject: Why does Calcite fluoresce blue?? |
|
|
| What makes calcite fluoresce blue under short wave? What trace element? What impurity?
|
|
| Back to top |
|
 |
Mike Wood

Joined: 16 Dec 2010
Posts: 456
Location: Northern England



|
 Posted: Nov 10, 2014 17:45 Post subject: Re: Why does Calcite fluoresce blue?? Posted: Nov 10, 2014 17:45 Post subject: Re: Why does Calcite fluoresce blue?? |
|
|
I have read (maybe an outdated source) that 'Iceland Spar' (presumably from Iceland?), sometimes shows a 'dull, blue luminescence' which was thought to have been caused by defect centres in the crystal lattice.
Calcite normally fluoresces yellow/orange/red and is usually caused by a small amount of Mn2+ substituting; in the range of a few hundred to a few thousand ppm. Apparently some REE such as Dy3+ and Sm3+ substituting in small amounts can produce a very similar colour range.
However, this info is over 25 years old and it is very likely there are more explanations available these days!
Mike
_________________
Rock basher |
|
| Back to top |
|
 |
Pete Modreski
Site Admin

Joined: 30 Jul 2007
Posts: 709
Location: Denver, Colorado



|
 Posted: Nov 14, 2014 18:08 Post subject: Re: Why does Calcite fluoresce blue?? Posted: Nov 14, 2014 18:08 Post subject: Re: Why does Calcite fluoresce blue?? |
|
|
And, my understanding is that it is high Europium (Eu2+) that causes the blue fluorescence.
Pete
|
|
| Back to top |
|
 |
Mark Ost

Joined: 18 Mar 2013
Posts: 516
Location: Virginia Beach



|
 Posted: Nov 17, 2014 06:45 Post subject: Re: Why does Calcite fluoresce blue?? Posted: Nov 17, 2014 06:45 Post subject: Re: Why does Calcite fluoresce blue?? |
|
|
| Calcium is not intrinsically fluorescent and requires activators such as Uranium or Europium. I have the typical red fluorescing calcite, but also green, more rare due to uranium content, and some yellow. My Icelandic spar shows no trace of color or afterglow under any frequency of UV.
|
|
| Back to top |
|
 |
Parvin
Joined: 27 Aug 2014
Posts: 22
Location: Georgia


|
 Posted: May 19, 2015 10:46 Post subject: Re: Why does Calcite fluoresce blue?? Posted: May 19, 2015 10:46 Post subject: Re: Why does Calcite fluoresce blue?? |
|
|
Hi Pete,
I have tried to use Eu+2 in order to make calcite fluoresce blue, but it didn't work out. Also, Eu is very expensive, Do you have any idea like what other activators cause calcite emits blue color?
Thanks so much.
Parvin
|
|
| Back to top |
|
 |
Rei
Joined: 09 Apr 2014
Posts: 228
Location: Höfuðborgarsvæði



|
 Posted: May 19, 2015 11:59 Post subject: Re: Why does Calcite fluoresce blue?? Posted: May 19, 2015 11:59 Post subject: Re: Why does Calcite fluoresce blue?? |
|
|
Parvin: What exactly are you doing with europium? Just dipping the calcite in it? Colors are due to small amounts of impurities in the crystal lattice (or defects in the lattice) - they're not surface treatments.
For what it's worth, I find a lot of massive calcite on my land in Iceland, and I've never found a specimen that fluoresces under UV (I've tried two frequencies). I always do my tests in the laundry room, and bleached white socks kick off a lot more glow than my calcite ;)
Sometimes a specimen just is what it is. There are all sorts of ways you can handle specimens, and sometimes they can make a world of difference. But a lot of things about a piece just can't be changed. If you want to make glowing calcite rather than finding it, you're going to need to grow it from scratch. Or paint it with glow-in-the-dark paint. ;) Hey, if you wanted to shell out the money you could use tritium paint, that'd be pretty awesome - it'd look like something straight out of a comic book ;)
|
|
| Back to top |
|
 |
lluis
Joined: 17 Nov 2006
Posts: 715


|
 Posted: May 19, 2015 12:07 Post subject: Re: Why does Calcite fluoresce blue?? Posted: May 19, 2015 12:07 Post subject: Re: Why does Calcite fluoresce blue?? |
|
|
Hi, Parvin
For what I found, blue color seems due to Eu2+ and Eu3+, acting cerium as activator....
So, probably you should use the three elements
Maybe you find this article interesting for your purposes....
https://www.fluomin.org/galeriespectre/spectre.php?lg=fr&name=CALCITE
(link normalized by FMF)
With best wishes
Lluís
|
|
| Back to top |
|
 |
Josele

Joined: 10 Apr 2012
Posts: 410
Location: Tarifa, Spain



|
 Posted: May 19, 2015 12:41 Post subject: Re: Why does Calcite fluoresce blue?? Posted: May 19, 2015 12:41 Post subject: Re: Why does Calcite fluoresce blue?? |
|
|
| lluis wrote: | | ... blue color seems due to Eu2+ and Eu3+, acting cerium as activator... |
In related graphics in fluomin.org can see that europium trivalent gives three peaks at about 590, 620 and 700 nm, which match with red, not blue. There is not peaks of Eu2+ in these graphics, bivalent europium is not listed as calcite activator in fluomin.org.
Cerium has the peak at 320 nm, which is into not visible UV range.
According to The nature of unusual luminescence in natural calcite CaCO3 by M. Gaft et al., activators of the uncommon blue luminiscence in calcite are still uncertain.
Parvin, can you explain how are you trying to add Eu impurities to calcite?
_________________
Josele |
|
| Back to top |
|
 |
Parvin
Joined: 27 Aug 2014
Posts: 22
Location: Georgia


|
 Posted: May 19, 2015 13:03 Post subject: Re: Why does Calcite fluoresce blue?? Posted: May 19, 2015 13:03 Post subject: Re: Why does Calcite fluoresce blue?? |
|
|
| Thanks, I will do.
|
|
| Back to top |
|
 |
Parvin
Joined: 27 Aug 2014
Posts: 22
Location: Georgia


|
 Posted: May 19, 2015 13:09 Post subject: Re: Why does Calcite fluoresce blue?? Posted: May 19, 2015 13:09 Post subject: Re: Why does Calcite fluoresce blue?? |
|
|
Josele,
So, you are saying that even with those three activators, it won't be possible to get the blue color? At what wavelength we should see the blue color?
Thanks.
Parvin
P.S. I am mixing the Eu doped in solution of chloride with ammunium carbonate in order to precipitate calcium carbonate.
|
|
| Back to top |
|
 |
lluis
Joined: 17 Nov 2006
Posts: 715


|
 Posted: May 19, 2015 13:32 Post subject: Re: Why does Calcite fluoresce blue?? Posted: May 19, 2015 13:32 Post subject: Re: Why does Calcite fluoresce blue?? |
|
|
Hi, Josele
I am not in fluorescence... Just chemist...
But, even with what you say, seems that in the article the blue calcite has the two states Europium. And the cerium.
Cerium is just the activator: it absorbs UV and transmits energy to Europium...
No color, no fluorescence... Just transmitting energy from UV to Eu... So, no Ce, no fluorescence....
Hi, Parvin: if you use only Eu, without activator, maybe you would not see fluorescence because no energy is transmitted to Eu.
By the way, the precipitation, seems that should be done at 70ºC, and precipitate should be treated thermally (in one I read at 1000ºC in CO2 atmosphere....).
Then, maybe you simply do not fit the required conditions....: thermal treatment and activator...
With best wishes
Lluís
|
|
| Back to top |
|
 |
Parvin
Joined: 27 Aug 2014
Posts: 22
Location: Georgia


|
 Posted: May 19, 2015 13:35 Post subject: Re: Why does Calcite fluoresce blue?? Posted: May 19, 2015 13:35 Post subject: Re: Why does Calcite fluoresce blue?? |
|
|
Hi Lluís,
Could you please tell me what article you are exactly referring?
Thank you.
Parvin
|
|
| Back to top |
|
 |
Josele

Joined: 10 Apr 2012
Posts: 410
Location: Tarifa, Spain



|
 Posted: May 19, 2015 14:01 Post subject: Re: Why does Calcite fluoresce blue?? Posted: May 19, 2015 14:01 Post subject: Re: Why does Calcite fluoresce blue?? |
|
|
Will see blue color when emission is in 450 - 500 nm range
In the abstract of the cited article about blue luminescence you can read: ... Both centers have spectral-kinetic properties very unusual for mineral luminescence, which in combination with extremely low impurity concentrations prevent their identification with specific impurity related emission. The most likely explanation of these observations may be the presence of radiation-induced luminescence centers.
If Michael Gaft doesn't know it, who knows it?
I'm curious of a more detailed explanation of your system to obtain doped calcite.
Can you describe the procedure? Did you get any results? Can you post some photos here?
| Description: |
|
| Viewed: |
31175 Time(s) |
|
_________________
Josele |
|
| Back to top |
|
 |
lluis
Joined: 17 Nov 2006
Posts: 715


|
 Posted: May 19, 2015 14:31 Post subject: Re: Why does Calcite fluoresce blue?? Posted: May 19, 2015 14:31 Post subject: Re: Why does Calcite fluoresce blue?? |
|
|
Parvin, the article is the one that I placed in my first message...
Long, besides.
Josele: Don is as human as us. He could be right...or he could be wrong.
In complex theory, were many, till last that seems right. Were the predecessors wrong? No, they just didn't have all the info.
When the wave function was generated by Schrödinger, Bohr said that it was the reality, and Einstein said that it was a way to explain reality....
As many heads, as many huts....
Anyway, I am fond to remember that a young lady was the one that showed that skeletal galenas were fake...when they have been sold by many seasoned experts....
So...
For colors..: Pt uses to be slightly yellow and with chloride ions, strong brown. But a cis-platinum is green..... (complex theory....).
Europium could be whatever. But if in calcite, in the right place, it creates the blue fluorescence.... As I said, if not wrong in such article, a blue fluorescent calcite had Eu2+, Eu3+ and cerium (as activator).
More.... Pb is in color...But amazonite is green due to Pb (color centers due to faults in crystal net due to different sizes....)
Then, nothing surprises me..... : things are and explanations, well, our work (the humans work, I mean)
With best wishes
Lluís
|
|
| Back to top |
|
 |
Josele

Joined: 10 Apr 2012
Posts: 410
Location: Tarifa, Spain



|
 Posted: May 19, 2015 19:41 Post subject: Re: Why does Calcite fluoresce blue?? Posted: May 19, 2015 19:41 Post subject: Re: Why does Calcite fluoresce blue?? |
|
|
I've been checking all calcites in my collection looking for a blue fluorescence, without result. About a third part showed a significant fluorescence. In this benchmarking of some representative specimens, we can see a small part of the diversity of luminescence in calcite, due to different activators, co-activators and combinations, and this gives an idea of the complexity.
| Description: |
1 - Halogen
2 - UVA (385 nm, filtered)
3 - UVC (255 nm, filtered)
Big piece at left has a mild blue-green-whitish fluorescence both under LW / SW
Second piece is Sulphur and calcite, which looks green-whitish both under LW / SW
Big rhombohedron is inert both under LW / SW
Piece on the right is a semi-dissolved calcite rounded by feldspar. This calcite is almost inert with LW but glows in dark red under SW
Cluster on base at left emits in red both under LW / SW
Second cluster at center is almost inert in LW and looks somewhat red under SW
The two generations of calcite in speleothem have a strong differentiated fluorescence. In this case activators are organic compounds.
Cobaltoan calcite is nearly dead under LW and revives strong with SW
Small rhombohedron at left looks dull red in LW and turns to orange with SW
Tabular crystal increases his red brightness from LW to SW
Small dogtooth calcite at front is the brightest piece, whitish red under LW and pure white with SW
Small rhombohedron at right that looks red both under LW / SW is accompanied by something blue, at last! what a surprise! After closer inspection turns to be fluorite. This piece has also galena, so is probable Pb is acting as coactivator under SW, emiting LW which amplifies other activator responsible of red fluorescence. |
|
| Viewed: |
31129 Time(s) |

|
_________________
Josele |
|
| Back to top |
|
 |
Pete Modreski
Site Admin

Joined: 30 Jul 2007
Posts: 709
Location: Denver, Colorado



|
 Posted: May 19, 2015 21:49 Post subject: Re: Why does Calcite fluoresce blue?? Posted: May 19, 2015 21:49 Post subject: Re: Why does Calcite fluoresce blue?? |
|
|
Josele, that's a nice assortment of calcites and a nice photo of them.
So, as you probably know, the blue-fluorescing calcite is very much the exception among calcites, even among fluorescent calcites. We often call it "Terlingua-type" calcite, and it has the unusual suite of properties of blue fluorescence SW, pink LW, and very bright and long-lived phosphorescence after the SW exposure. And there are only a few known localities where calcite with these properties occurs; the main ones being Terlingua TX, one locality in Mexico, and Hope, Indiana (there may be a few others, less well know). And my understanding is that it's divalent europium, Eu2+, that emits this blue fluorescence, though there may indeed by other rare-earth elements present that help absorb the UV light that is re-emitted by the europium (and thus, they would be co-activators). But I don't think there exists any hard data as to whether or not there are such co-activators, and just what they are or what their role may be. (There's still a lot of research left to be done to really understand this sort of thing!)
And of course, the only way to synthesize such calcite, would be to precipitate calcite from a solution that contains europium salts (and varying the oxidation state of the europium, Eu2+ or Eu3+, is another variable that one could experiment with), and see if the presence of other REE, or the temperature at which the calcite was deposited, etc., had any effects on the fluorescence. This type of synthesis experiment, unless done under high pressure hydrothermal conditions, would only produce a very fine-grained powdered form of calcite, not macroscopic crystals.
I'm about run out of ideas for a response, after saying this!
|
|
| Back to top |
|
 |
Josele

Joined: 10 Apr 2012
Posts: 410
Location: Tarifa, Spain



|
 Posted: May 20, 2015 18:21 Post subject: Re: Why does Calcite fluoresce blue?? Posted: May 20, 2015 18:21 Post subject: Re: Why does Calcite fluoresce blue?? |
|
|
While it is true that some information talk about Eu as activator of blue fluorescence in calcite, I can not found any serious paper or reference confirming this assertion. Will appreciate any help to find it.
At the moment, after re-reading all relative to calcite in Luminescence Spectroscopy of Minerals and Materials of M. Gaft et al., must insist in uncertainty of which activator causes blue fluorescence in calcite.
Is well stablished that Eu2+ can activate blue fluorescence in fluorite, apatite, Baryte, danburite, feldspars, anhydrite, zoisite and charoite (page 331, Table 12.1, REE luminescence centers detected in minerals, where calcite is not listed in Eu2+ activated minerals). This does not mean that can't occur, just that the authors they have not found it.
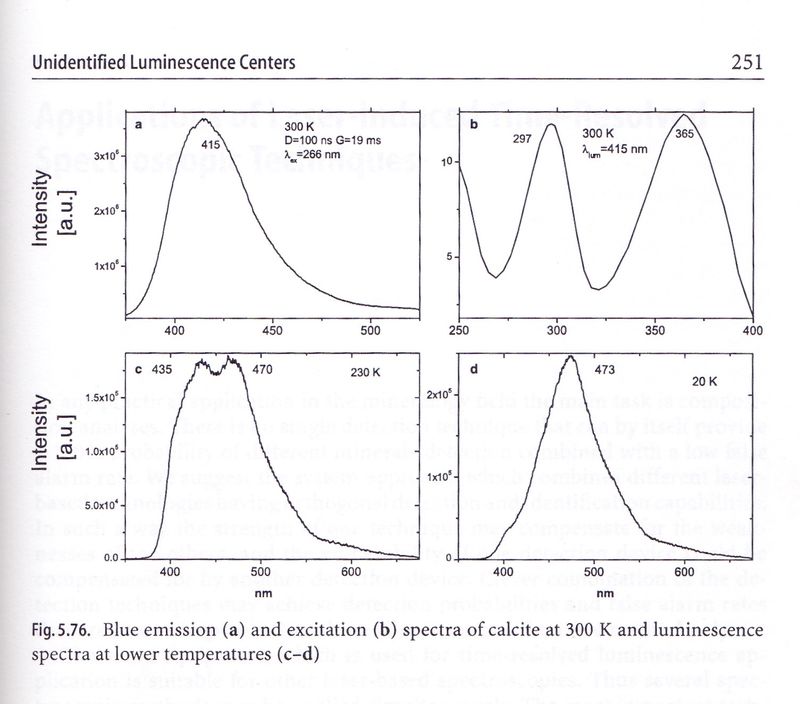
In chapter Unidentified Luminiscence Centers (paragraph 5.11.4 Calcite, page 250), authors say: "Under short-weved UV lamp excitation (254 nm) visually observed luminiscence of calcite is violet-blue with very long phosphorescence time of several seconds. ..." whose characteristics closely resemble to Terlingua-type calcite. After a discussion of tests results they don't arrive to a consistent identification of the activator and concluded "Additional research needed in order to clarify this problem".
In other hand, regardless of activators, I think, as Pete, that must be not easy to crystalize fluorescent calcite with home-made systems. That's why I encourage Parvin to explain his method.
| Description: |
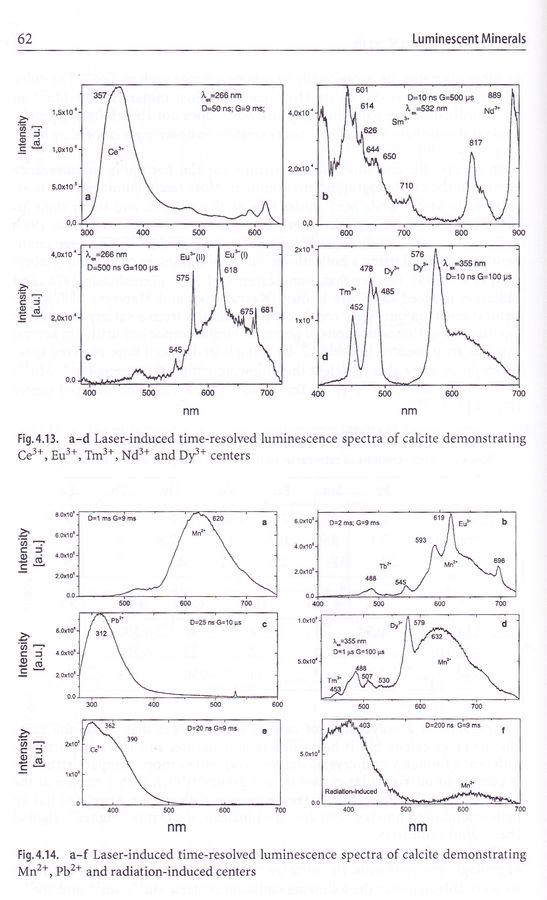
Luminiscence spectra of calcite activators
Eu3+ and Mn2+, which is most common of them and also the most intense, glows in red range while Ce3+ and Pb2+ emits in the UV range.
Note that none of them have the peak in 450-500 nm blue range, only radiation-induced center shows some low intensity in violet-blue range.
image © M. Gaft et al., Luminescence Spectroscopy of Minerals and Materials, page 60 |
|
| Viewed: |
30925 Time(s) |
|
| Description: |
Luminiscence spectra of calcite with unidentified activator.
The emmision peak is centered in violet-blue range between 450 and 500 nm depending of temperature.
image © M. Gaft et al., Luminescence Spectroscopy of Minerals and Materials, page 251 |
|
| Viewed: |
30919 Time(s) |
|
_________________
Josele |
|
| Back to top |
|
 |
Pete Modreski
Site Admin

Joined: 30 Jul 2007
Posts: 709
Location: Denver, Colorado



|
 Posted: May 21, 2015 12:02 Post subject: Re: Why does Calcite fluoresce blue?? Posted: May 21, 2015 12:02 Post subject: Re: Why does Calcite fluoresce blue?? |
|
|
| Josele, I want to retract/amend my statement that europium (Eu2+) is responsible for the blue fluorescence of some calcite. I agree with what you've quoted; there really is no hard evidence for this, it is just a statement that was based on supposition. I shouldn't have been making that claim without checking out the sources (if there even were any!). Thank you for pointing this out.
|
|
| Back to top |
|
 |
Josele

Joined: 10 Apr 2012
Posts: 410
Location: Tarifa, Spain



|
 Posted: May 21, 2015 15:32 Post subject: Re: Why does Calcite fluoresce blue?? ...or white??? Posted: May 21, 2015 15:32 Post subject: Re: Why does Calcite fluoresce blue?? ...or white??? |
|
|
Thank you, Pete, for relating Parvin's question with Terlingua-type calcite. My knowledge is very limited but I became curious and I was lucky for having the right consulting book.
Let me take this thread to another question about fluorescence. I have understood that each activator emits in a determined range which corresponds to a color, then, what causes white fluorescence? White is the addition of all visible colors together. When we see a white fluorescence, that means there is three or more activators emitting in a range that added to others create white light?
Very strong emission in any color also is seen white, but usually can see the color in the edges of the piece. However sometimes fluorescence is pure white, how does it work?
Comments will be appreciated, thanks for your interest.
| Description: |
1 - Halogen
2 - UVA (385 nm, filtered)
3 - UVC (255 nm, filtered)
Three calcites with different response under LW and SW
I see the small dog-tooth at left glowing really white-white but maybe you can perceive a hue... |
|
| Viewed: |
30798 Time(s) |

|
_________________
Josele |
|
| Back to top |
|
 |
CranCowan
Joined: 28 Sep 2016
Posts: 18
Location: Hines



|
 Posted: Sep 30, 2016 13:54 Post subject: Re: Why does Calcite fluoresce blue?? Posted: Sep 30, 2016 13:54 Post subject: Re: Why does Calcite fluoresce blue?? |
|
|
The most commonly blue fluorescing calcite (under SW) is probably the so called Terlingua-type.
It's activator is thought to be organic. (Robbins, Graft ...)
|
|
| Back to top |
|
 |
|


