| View previous topic :: View next topic |
| Author |
Message |
Tobi
Site Admin

Joined: 07 Apr 2009
Posts: 4108
Location: Germany



|
 Posted: Nov 27, 2023 09:17 Post subject: Fluorite crystallography? Posted: Nov 27, 2023 09:17 Post subject: Fluorite crystallography? |
|
|
Dear fellow collectors,
when I posted this Naica fluorite, an interesting discussion started about the crystal morphology and how to name this kind of form in the cubic system. I admit that I am a total layman in this subject, so I start this discussion as a new topic. Maybe someone with a lot of knowledge (maybe Pete Richards?) can help here ...
Photos are attached, the original discussion is here:
https://www.mineral-forum.com/message-board/viewtopic.php?p=82190#82190
| Mineral: | Fluorite, Sphalerite |
| Locality: | | Naica Mine, Naica, Municipio Saucillo, Chihuahua, Mexico |  |
|
| Dimensions: | Specimen height 8 cm |
| Description: |
|
| Viewed: |
8384 Time(s) |

|
| Mineral: | Fluorite, Sphalerite |
| Locality: | | Naica Mine, Naica, Municipio Saucillo, Chihuahua, Mexico |  |
|
| Dimensions: | Specimen height 8 cm |
| Description: |
| Photo © Martin Gruell / viaMineralia |
|
| Viewed: |
8420 Time(s) |

|
|
|
| Back to top |
|
 |
Roger Warin

Joined: 23 Jan 2013
Posts: 1176



|
 Posted: Nov 27, 2023 10:05 Post subject: Re: Fluorite crystallography? Posted: Nov 27, 2023 10:05 Post subject: Re: Fluorite crystallography? |
|
|
cuboctahedron
Cheers Tobi.
|
|
| Back to top |
|
 |
Roger Warin

Joined: 23 Jan 2013
Posts: 1176



|
 Posted: Nov 27, 2023 10:12 Post subject: Re: Fluorite crystallography? Posted: Nov 27, 2023 10:12 Post subject: Re: Fluorite crystallography? |
|
|
sorry
Rhombododecahedron as copper.
|
|
| Back to top |
|
 |
Peter Megaw
Site Admin

Joined: 13 Jan 2007
Posts: 963
Location: Tucson, Arizona



|
 Posted: Nov 27, 2023 10:12 Post subject: Re: Fluorite crystallography? Posted: Nov 27, 2023 10:12 Post subject: Re: Fluorite crystallography? |
|
|
Nice fluorites Tobi! Classic Naica.
A high percentage of Naica fluorites show this combination of the cube (001) and octahedron (111)...commonly called a cube-octahedron...or cube-octahedral.
To sort this out yourself...mentally take the 6 faces that are "square" and extend them until they meet...you'll get 6 identical faces at 90 degrees to each other...a cube. Then do the same with the 8 hexagonal faces and you'll get the octahedron.
One of the cool things about Naica fluorites (and those from other skarn deposits, which tend to have remarkably similar fluorites worldwide) is the common composite octahedrons built up of multiple repetitions of the cube-octahedron. The two images above show an obvious and less obvious variations on this theme. Note that the size/expression of the cube versus the octahedron varies Yes, there are a few extra forms present in the first example just to keep things interesting!
| Mineral: | Fluorite |
| Locality: | | Naica, Municipio Saucillo, Chihuahua, Mexico |  |
|
| Dimensions: | 3 cm |
| Description: |
| Octahedron composed of repeated cube-octahedrons |
|
| Viewed: |
8344 Time(s) |

|
| Locality: | | Naica, Municipio Saucillo, Chihuahua, Mexico |  |
|
| Dimensions: | 8cm |
| Description: |
| Octahedron built up of tiny cube-octahedrons |
|
| Viewed: |
8395 Time(s) |

|
_________________
Siempre Adelante! |
|
| Back to top |
|
 |
Peter Megaw
Site Admin

Joined: 13 Jan 2007
Posts: 963
Location: Tucson, Arizona



|
 Posted: Nov 27, 2023 10:13 Post subject: Re: Fluorite crystallography? Posted: Nov 27, 2023 10:13 Post subject: Re: Fluorite crystallography? |
|
|
Tobi...could I get high-rez versions of these images with permission to publish? I am working on an article on Naica and these are worth considering for inclusion.
_________________
Siempre Adelante! |
|
| Back to top |
|
 |
Roger Warin

Joined: 23 Jan 2013
Posts: 1176



|
 Posted: Nov 27, 2023 10:18 Post subject: Re: Fluorite crystallography? Posted: Nov 27, 2023 10:18 Post subject: Re: Fluorite crystallography? |
|
|
Dowty Eric, Richards Peter R. - Mode d’emploi
Shape - A computer program for drawing crystals -
Macintosh version 4.0 (1991-1993)
|
|
| Back to top |
|
 |
James Catmur
Site Admin

Joined: 14 Sep 2006
Posts: 1346
Location: Cambridge



|
 Posted: Nov 27, 2023 11:34 Post subject: Re: Fluorite crystallography? Posted: Nov 27, 2023 11:34 Post subject: Re: Fluorite crystallography? |
|
|
These always intrigue me, especially the centre line 'phantom' in each crystal
One can always consult the "Crystal Forms of Fluorite" by Eddy van Der Meersche, as that seems very exhaustive
| Mineral: | Fluorite |
| Locality: | | Jaimina Mine, Obdulia vein, Caravia mining area, Trechorio, Carrales, Caravia, Comarca Oriente, Principality of Asturias (Asturias), Spain |  |
|
| Dimensions: | Crystal measures 3 cm x 3cm x 3 cm |
| Description: |
|
| Viewed: |
8317 Time(s) |

|
|
|
| Back to top |
|
 |
Carles Millan
Site Admin

Joined: 05 May 2007
Posts: 1471
Location: Catalonia



|
 Posted: Nov 27, 2023 11:38 Post subject: Re: Fluorite crystallography? Posted: Nov 27, 2023 11:38 Post subject: Re: Fluorite crystallography? |
|
|
| In my humble opinion (I'm far from an expert of crystallography), those crystals (the ones from Tobi) are the combination of {100} (cube) and {110} (rhombododecahedron), showing a total of 18 faces.
|
|
| Back to top |
|
 |
Bob Morgan
Joined: 18 Jan 2018
Posts: 228
Location: Savannah, Georgia



|
 Posted: Nov 27, 2023 11:50 Post subject: Re: Fluorite crystallography? Posted: Nov 27, 2023 11:50 Post subject: Re: Fluorite crystallography? |
|
|
I'm sorry, I have to differ with Peter. These are cube and dodecahedral forms in combination.
It is a common problem to distinguish them from the cube-octahedral crystals.
On your two crystals the dodecahedral faces run parallel to striations on the cube face. On Peter's 'Mayan Temple" the smaller crystals are running down what would be an octahedral edge but have cube-dodecahedral faces that lie flat across what would be octahedral edges. On his dodecahedron, the small flat faces along the edges are dodecahedral.
I like Pete's suggestion of imaging the expansion of faces until they become the whole crystal. If you do that with dodecahedral faces, the shape of a dodecahedral garnet will be the result, with 4 faces terminating at the top and 4 at the bottom, and four faces around the middle.
I've worked on and off on a spotters guide to face identification for the beginner. 'Just haven't seen it through wondering if there is enough interest in such.
|
|
| Back to top |
|
 |
Bob Morgan
Joined: 18 Jan 2018
Posts: 228
Location: Savannah, Georgia



|
 Posted: Nov 27, 2023 11:53 Post subject: Re: Fluorite crystallography? Posted: Nov 27, 2023 11:53 Post subject: Re: Fluorite crystallography? |
|
|
| Another hint: In both cases there are four faces bordering cube faces. But they have differing orientation to the cube faces. Octahedral faces will develop off the corners of cube faces, dodecahedral off the edges of cube faces.
|
|
| Back to top |
|
 |
Bob Morgan
Joined: 18 Jan 2018
Posts: 228
Location: Savannah, Georgia



|
 Posted: Nov 27, 2023 11:59 Post subject: Re: Fluorite crystallography? Posted: Nov 27, 2023 11:59 Post subject: Re: Fluorite crystallography? |
|
|
Nice piece and a good photo.
These are etched approximations of hexoctahedral faces on the cube, for which Jaimina stands out.
|
|
| Back to top |
|
 |
Tobi
Site Admin

Joined: 07 Apr 2009
Posts: 4108
Location: Germany



|
 Posted: Nov 27, 2023 13:19 Post subject: Re: Fluorite crystallography? Posted: Nov 27, 2023 13:19 Post subject: Re: Fluorite crystallography? |
|
|
| Peter Megaw wrote: | | Tobi...could I get high-rez versions of these images with permission to publish? I am working on an article on Naica and these are worth considering for inclusion. |
Hi Peter, the photo from Rudi Watzl with its 1783 x 1260 px is the largest I have at the moment. I just wrote Rudi and asked if he has a larger version, I'm sure he would give you the permission. If he has no larger version, I can try to make my own photos. I give you any permission you need, but don't expect my photos to be good enough :-/
I'll let you know if a larger (and better) version is possible ;-)
|
|
| Back to top |
|
 |
Pete Richards
Site Admin

Joined: 29 Dec 2008
Posts: 828
Location: Northeast Ohio



|
 Posted: Nov 27, 2023 13:49 Post subject: Re: Fluorite crystallography? Posted: Nov 27, 2023 13:49 Post subject: Re: Fluorite crystallography? |
|
|
Toby’s post presents a question that puzzles many collectors, and the answer is not always immediately evident. We have a cube (100) modified by the octahedron (111) or the rhombic dodecahedron (110), but which is it? Even after working with crystal morphology for years, I have to look twice and argue with myself before I am sure of my answer. Roger illustrates the dilemma with his two successive postings, choosing one and then the other interpretation.
Peter offers one way of analyzing the situation, which is to visualize the crystal as it would be with only one of the forms. If you can do this, it can lead to the right answer. The octahedron has 8 faces, which cut off the corners of the cube; the dodecahedron has 12, which cut off the edges. But as Bob Morgan points out, in crystals with the habit of Tobi’s, each cube face is surrounded by four faces of the other form, whether it is an octahedron or a dodecahedron. Doing the visualization exercise is especially difficult in a photograph which is oriented looking nearly straight toward a cube face (i.e. looking down a four-fold axis). After a lot of looking, I think I disagree with Peter's conclusion....
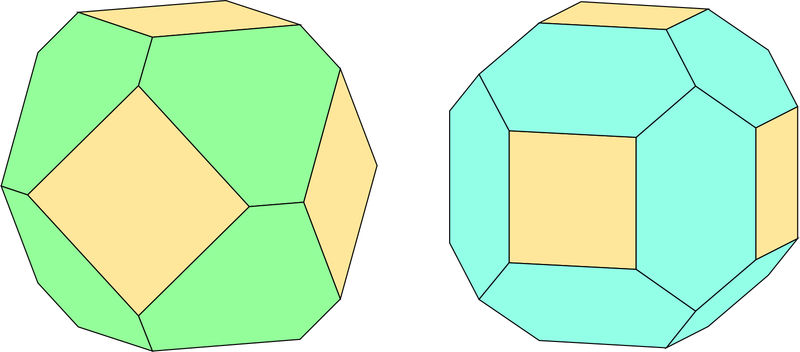
Here are some further thoughts about how to tell these two form combinations apart. This discussion presumes that the faces are about equally developed (if not, the answer is usually obvious), and that the cube faces are completely bounded by faces of one of the other forms, but not both. The first drawing shows the two possibilities – with cube faces colored tan, octahedron faces colored green, and dodecahedral faces colored blue.
In these drawings, a cube face is oriented more or less to the front (rotated a bit to the left to provide perspective). Both crystals have a set of four faces sloping back away from this face. Behind these faces are more cube faces in the cube-octahedral crystal, but a combination of cube and dodecahedral faces in the cube-dodecahedron. Seven faces are visible ( out of 14) in the left crystal, but nine (of 18) are visible on the right.
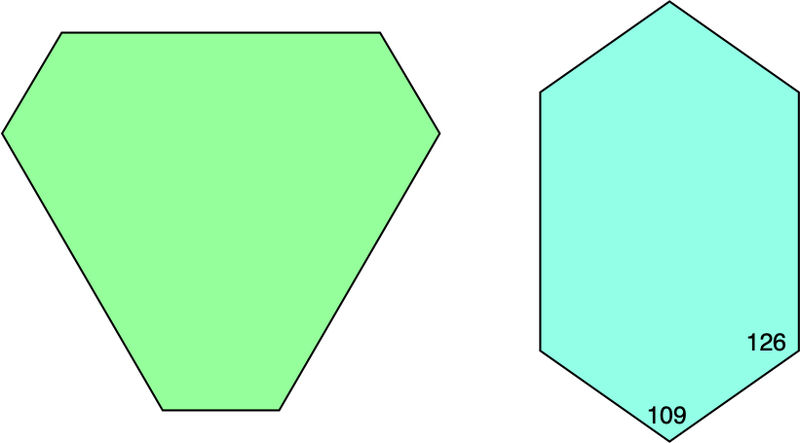
The cube faces can be recognized as squares (or rectangles, if the crystal development is not perfect). The shapes of the modifying faces are diagnostic. The second set of drawings shows one face of each type, viewed looking straight down onto them. The octahedral face has three-fold symmetry, reflecting its position on the crystal perpendicular to the three-fold symmetry axis. It has six edges (given the stated assumptions). Typically there are three long ones alternating with three shorter ones, but a perfect hexagon can occur if the contributing faces are of the right sizes. Each corner is a 120° angle. The dodecahedral face also has six faces, but there are two faces on each end and longer faces on the sides. The symmetry is two-fold rather than three-fold, again reflecting the orientation of dodecahedral faces perpendicular to a two-fold symmetry axis. The angles at the corners are not the same and none is 120°, though this distinction is hard to see except perhaps at the angle between pairs of shorter faces.
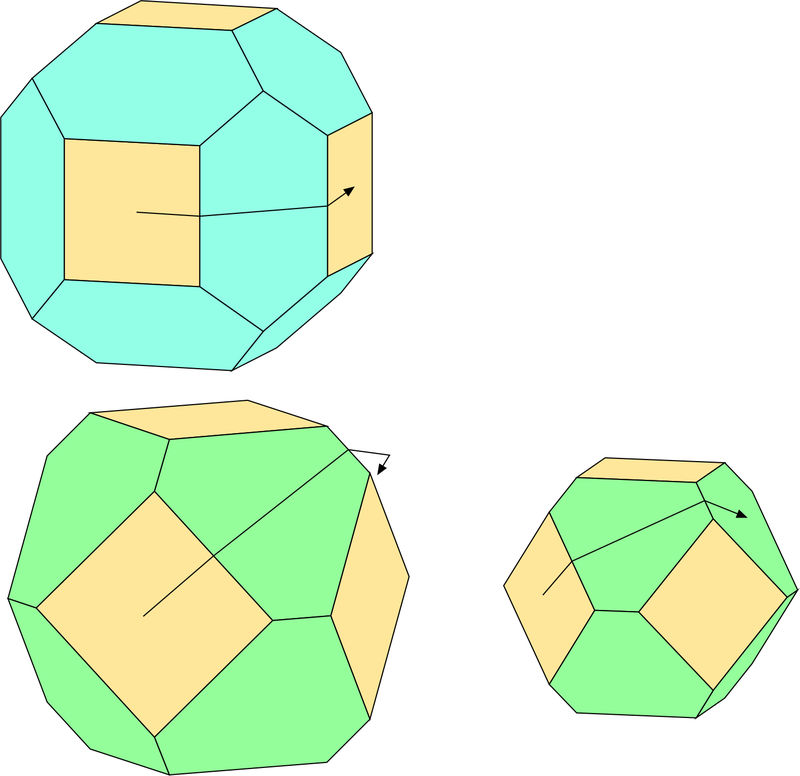
Another way of making sense of these crystals is one that was first pointed out to me by Herwig Pelkmans. If one imagines staring in the middle of a cube face, “walking” to an edge along a line perpendicular to the edge, then continuing across the modifying face until another edge is reached and crossing that edge into a new face, the identity of the new face is diagnostic of the modifying face. If the modifying face belongs to a dodecahedron, you will have reached another cube face. If it belongs to an octahedron, you will reach another octahedron and need to go across it to reach the next cube face. On the larger drawing of the cube-octahedron, the destination face is not shown in the standard orientation. The smaller drawing is of a crystal rotated to the left through 40°, which allows the complete path to be shown.
A different way of expressing the same neighboring-face relationships is that on a cube-dodecahedron you cross cube edges to reach another cube face in two steps, but in a cube-octahedron you go to the corners of the cube faces (and “walk the line” between two octahedral faces).
Some will immediately see that if that edge was beveled by a thin face, it would be the face of a dodecahedron. But that would present us with a crystal composed of three forms, and a much longer discussion!
OK, I'd better give my answer. One addition piece of information from the symmetry of this system: if you can identify a corner of the crystal, and the crystal is cube-octahedral, there will be a single octahedral face there cutting off the corner. If the crystal is cube-dodecahedral, three dodecahedral faces will meet at the corner. I think both of Tobi's photos show this latter pattern quite clearly.
| Mineral: | Fluorite (computer generated) |
| Description: |
|
| Viewed: |
8263 Time(s) |
|
| Description: |
|
| Viewed: |
8263 Time(s) |
|
| Description: |
|
| Viewed: |
8271 Time(s) |
|
_________________
Collecting and studying crystals with interesting habits, twinning, and epitaxy |
|
| Back to top |
|
 |
Tobi
Site Admin

Joined: 07 Apr 2009
Posts: 4108
Location: Germany



|
 Posted: Nov 27, 2023 16:51 Post subject: Re: Fluorite crystallography? Posted: Nov 27, 2023 16:51 Post subject: Re: Fluorite crystallography? |
|
|
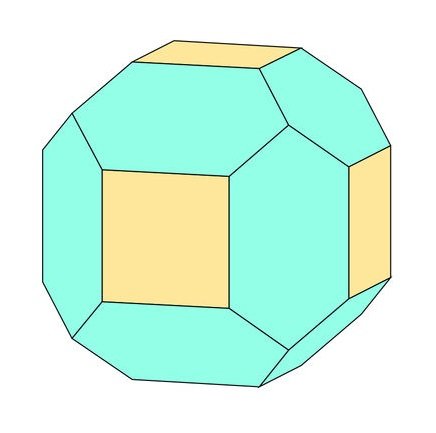
That is exactly what the crystals look like - thanks Pete :-)
| Mineral: | Fluorite (computer generated) |
| Description: |
| Pete's drawing with 18 faces matches exactly the crystal habit of my fluorite specimen from Naica |
|
| Viewed: |
8162 Time(s) |
|
|
|
| Back to top |
|
 |
Carles Millan
Site Admin

Joined: 05 May 2007
Posts: 1471
Location: Catalonia



|
 Posted: Nov 27, 2023 17:05 Post subject: Re: Fluorite crystallography? Posted: Nov 27, 2023 17:05 Post subject: Re: Fluorite crystallography? |
|
|
| So it is {100}{110}.
|
|
| Back to top |
|
 |
Pete Richards
Site Admin

Joined: 29 Dec 2008
Posts: 828
Location: Northeast Ohio



|
 Posted: Nov 28, 2023 08:35 Post subject: Re: Fluorite crystallography? Posted: Nov 28, 2023 08:35 Post subject: Re: Fluorite crystallography? |
|
|
| Carles Millan wrote: | | So it is {100}{110}. |
Yes, it is {100}{110}. I checked my small collection of Naica fluorites last night, and mine also have this habit.
An interesting extra is that some of them have an overall octahedral shape, like ones that Peter Megaw illustrated. In spite of the overall shape, the faces on the specimen are all cube or dodecahedral faces. It seems quite likely that there was a first generation that was octahedral, and the second generation of cube and dodecahedron overgrew it. I'll add a photo if I can get a decent one....
_________________
Collecting and studying crystals with interesting habits, twinning, and epitaxy |
|
| Back to top |
|
 |
Tobi
Site Admin

Joined: 07 Apr 2009
Posts: 4108
Location: Germany



|
 Posted: Nov 28, 2023 10:00 Post subject: Re: Fluorite crystallography? Posted: Nov 28, 2023 10:00 Post subject: Re: Fluorite crystallography? |
|
|
| Tobi wrote: | | Peter Megaw wrote: | | Tobi...could I get high-rez versions of these images with permission to publish? I am working on an article on Naica and these are worth considering for inclusion. |
I'll let you know if a larger (and better) version is possible ;-) |
Hi Peter, I just received an answer from Rudi Watzl's assistant Konrad, he sent me the largest version of the file that they have. Resolution is the same but the file size is much larger, I hope this image that I attached to this post is good enough for you?
Concerning the credits he wrote:
"Natürlich könnt ihr das Foto gerne auch veröffentlichen. Bitte bei den Credits „Foto: Saphira Minerals“ schreiben." ("Of course you are welcome to publish the photo. Please write “Photo: Saphira Minerals” in the credits.")
So please feel free to use it :-)
Regards
Tobi
| Mineral: | Fluorite, Sphalerite |
| Locality: | | Naica Mine, Naica, Municipio Saucillo, Chihuahua, Mexico |  |
|
| Dimensions: | Specimen height 8 cm |
| Description: |
|
| Viewed: |
8003 Time(s) |

|
|
|
| Back to top |
|
 |
Jesse Fisher

Joined: 18 Mar 2009
Posts: 629
Location: San Francisco



|
 Posted: Nov 28, 2023 11:08 Post subject: Re: Fluorite crystallography? Posted: Nov 28, 2023 11:08 Post subject: Re: Fluorite crystallography? |
|
|
Another Naica fluorite, which according to Pete's analysis would be cube-dodechahedral habit. From the find circa 2005.
| Mineral: | Fluorite on Sphalerite |
| Locality: | | Naica Mine, Naica, Municipio Saucillo, Chihuahua, Mexico |  |
|
| Dimensions: | 14x10x6 cm |
| Description: |
| cube-dodecahedral habit, with inclusions of what appears to be pyrite. |
|
| Viewed: |
7944 Time(s) |

|
|
|
| Back to top |
|
 |
Bob Morgan
Joined: 18 Jan 2018
Posts: 228
Location: Savannah, Georgia



|
 Posted: Nov 28, 2023 11:45 Post subject: Re: Fluorite crystallography? Posted: Nov 28, 2023 11:45 Post subject: Re: Fluorite crystallography? |
|
|
Pete,
Some of us call these 'Mayan Temple" habit fluorites. Yes, these are the result of smaller overgrowths on octahedral crystals. I have a specimen from Naica that has an octahedral phantom sprinkled with small galena crystals, that can be seen through clear cube faces at the terminations of the underlying octahedron. This particular specimen has overgrowths that are cubes with small dodecahedral faces and larger trapezohedral faces and the occasional small octahedral face.
The original octahedral stage is an indication of a higher temperature of formation, which is supported by the octahedral habit of the small galena crystals. Subsequent overgrowths then were lower temperature aligned deposits more characteristic of cube shapes.
'Myan Temple' habit fluorites come from other places. I've seen them from Dalnegorsk, China and even places in the US that I can't recall.
Larger overgrowths tend to be on the octahedral terminations with smaller ones down the octahedral edges and even smaller ones stepping all over underlying octahedral faces.
|
|
| Back to top |
|
 |
Pete Richards
Site Admin

Joined: 29 Dec 2008
Posts: 828
Location: Northeast Ohio



|
 Posted: Nov 28, 2023 13:40 Post subject: Re: Fluorite crystallography? Posted: Nov 28, 2023 13:40 Post subject: Re: Fluorite crystallography? |
|
|
| Bob Morgan wrote: | (snip)
Larger overgrowths tend to be on the octahedral terminations with smaller ones down the octahedral edges and even smaller ones stepping all over underlying octahedral faces. |
Bob's comments about overgrowth patterns are supported by the photos below of one of my Naica specimens. Peter Megaw's photos earlier also show the general pattern of overgrowth on an earlier octahedron - particularly the second one in which the overgrowth is only minimal.
The slower and somewhat chaotic overgrowth in the middles of the original octahedral faces leaves them depressed in comparison to the corners and edges adorned by the cube and dodecahedral faces.
| Mineral: | Fluorite |
| Locality: | | Naica, Municipio Saucillo, Chihuahua, Mexico |  |
|
| Dimensions: | 2 cm |
| Description: |
| View showing cube and dodecahedal overgrowths on a corner and edges of a former octahedral crystal. Natural light. |
|
| Viewed: |
7844 Time(s) |

|
| Description: |
|
| Viewed: |
7916 Time(s) |

|
| Mineral: | Fluorite |
| Locality: | | Naica, Municipio Saucillo, Chihuahua, Mexico |  |
|
| Description: |
| Top view with cube face highlighted. Artificial light. |
|
| Viewed: |
7902 Time(s) |

|
_________________
Collecting and studying crystals with interesting habits, twinning, and epitaxy |
|
| Back to top |
|
 |
|



