| View previous topic :: View next topic |
| Author |
Message |
Eduardoo
Joined: 01 Jun 2010
Posts: 72
Location: Quito


|
 Posted: Jun 02, 2010 22:12 Post subject: Chemical identification Posted: Jun 02, 2010 22:12 Post subject: Chemical identification |
|
|
Besides hardness, SG, HCl tests, streak, color, luster, and similar, what chemical analysis are available to the amateur mineralogist?
By available I mean analysis that do not cost a fortune and can reasonably be done at home.
Would you please provide some tips or direct me to some relevant source?
Thank you very much.
Eduardo.
|
|
| Back to top |
|
 |
Paul S

Joined: 20 Mar 2010
Posts: 79



|
 Posted: Jun 03, 2010 02:52 Post subject: Re: Chemical identification Posted: Jun 03, 2010 02:52 Post subject: Re: Chemical identification |
|
|
You can easily test for solubility, using different kinds of solvents, including a variety of acids.
If a mineral is soluble, you can see if it forms a precipitate if a chemical is added to the solution. Whether is forms a precipitate or not will show you with what chemical you are dealing.
Reactions can sometimes be seen when one chemical is added to another. There are too many possibilities to sum them all up, but if you think you know what chemical your mineral is, you can easily verify or disqualify it this way.
Flame colour tests can be helpful, but it is not always easy getting a good result. This test can show you what ions are present. If there are many different ones present you will not get a good result, but if there is one type of ion present in abundance, you will get a clear colour to identify it with.
And don’t forget smell as a chemical test. I don’t have a list of smells that belong to a mineral, but the smell of rotten eggs points to sulphur bearing minerals.
|
|
| Back to top |
|
 |
David Von Bargen
Joined: 09 Jul 2009
Posts: 41
Location: Milwaukee


|
 Posted: Jun 03, 2010 07:27 Post subject: Re: Chemical identification Posted: Jun 03, 2010 07:27 Post subject: Re: Chemical identification |
|
|
| The Mineralogical Record published a book "Mineral Identification: A Practical Guide for the Amateur Mineralogist" — by Donald B. Peck that is an excellent guide to tests that can be done at home.
|
|
| Back to top |
|
 |
Jordi Fabre
Overall coordinator of the Forum

Joined: 07 Aug 2006
Posts: 4899
Location: Barcelona



|
 Posted: Jun 03, 2010 07:58 Post subject: Re: Chemical identification Posted: Jun 03, 2010 07:58 Post subject: Re: Chemical identification |
|
|
And Eudardoo, as it seems to be that you can read Spanish language you can also visit this thread in the Spanish Forum -> https://www.foro-minerales.com/forum/viewtopic.php?t=154 where you will find a lot of basic information.
_________________
Audaces fortuna iuvat |
|
| Back to top |
|
 |
Eduardoo
Joined: 01 Jun 2010
Posts: 72
Location: Quito


|
 Posted: Jun 03, 2010 08:47 Post subject: Re: Chemical identification Posted: Jun 03, 2010 08:47 Post subject: Re: Chemical identification |
|
|
| Gracias amigos.
|
|
| Back to top |
|
 |
Luiz Menezes
Joined: 10 Dec 2009
Posts: 140
Location: Belo Horizonte


|
 Posted: Jun 03, 2010 11:44 Post subject: Re: Chemical identification Posted: Jun 03, 2010 11:44 Post subject: Re: Chemical identification |
|
|
Another interesting physical property that you can investigate is fluorescence; you need to buy an ultraviolet lamp, designed for use for minerals (with a short-wave an a long-wave source); it is normally not a conclusive test, but it can help to identify the presence of different phases in a non-crystallized specimen, and can be an important additional information, on many cases.
Can any of our friends at the Forum recommend me and Eduardoo some bibliography about a good database on fluorescent minerals?
Luiz
|
|
| Back to top |
|
 |
Antonio Alcaide
Site Admin

Joined: 23 Aug 2009
Posts: 314
Location: Spain



|
|
| Back to top |
|
 |
Gerhard Niklasch
Joined: 27 Mar 2009
Posts: 134
Location: Munich



|
 Posted: Jun 04, 2010 08:18 Post subject: Re: Chemical identification Posted: Jun 04, 2010 08:18 Post subject: Re: Chemical identification |
|
|
Indeed fluorescence can be immensely useful - not least in spotting that something unexpected is present on or in a specimen in the first place, which may be not at all conspicuous under visible light! I've made a habit of holding each and every new acquisition under the UV lamp however unlikely it may be, and I've had many surprises.
(NB short-wave vs. long-wave UV makes a difference: Ideally, get a lamp which is capable of providing either.)
The difficulty in using this as a diagnostic, though, is that only a minority of minerals (such as Scheelite) are intrinsically fluorescent. Many more (Fluorite, Calcite, Apatite,....) fluoresce due to impurities in trace amounts, and thus their response to UV can be quite different from one locality to another - sometimes it varies even from one zone to another in one and the same small crystal.
For an example where it's useful and usefully documented: The listing of minerals in the great, lavishly illustrated Långban book (D. Holtstam and J. Langhof, eds., 1999) goes to great lengths in pointing out fluorescence, which helps to recognize some of the many species occurring there which are otherwise hard to distinguish from other species. These hints may carry over, taken with a grain of salt, to other localities in the vicinity (Harstigen, Jakobsberg), and with a large dose of salt to other deposits of a broadly similar geochemistry (e.g. the Franklin district in New Jersey), but they won't necessarily generalize to material from elsewhere on the planet.
For a counterexample, look up Zincite on mindat. (In that case, the behavior varies drastically already within the Franklin district!)
Cheers, Gerhard
|
|
| Back to top |
|
 |
bugrock

Joined: 24 Nov 2008
Posts: 137
Location: Michigan


|
 Posted: Jun 04, 2010 22:25 Post subject: Re: Chemical identification Posted: Jun 04, 2010 22:25 Post subject: Re: Chemical identification |
|
|
Regarding UV response,
Having a SW and LW combined lamp works well for many specimens but sometime after purchase of that UV arrangement I picked up a MW lamp since in the fluorescent collecting sphere there are some specimens known to show better response in MW.
I also shine many pieces just to see what happens. What has been interesting is that for those minerals that are know for their SW response (maganocalcite is an example) the MW lamp very frequently shows more impressive, brighter fluorescence. This happened so often that I began to think that my MW lamp was simply putting out more light, an amplitude response rather than one of wavelength. Yet some rocks show the best response in SW, are weak in MW and little to nothing in LW. Recent lowly example I tried was an artifact rock, slag from old iron mines. Large amounts of this material wash up on some of the beaches of Lake Superior where it was dumped for many years. This bubbly/glassy material shows response in most pieces, great in SW...yellow to orange in many pieces, much weaker but same color in MW and little to nothing in LW.
Minority of pieces (20-30%) show no response.
For those interested in fluorescent minerals as a collectable group would advise a set up that can deliver all three wavelength ranges. For those who are just curious about how specimens respond a three wavelength unit (SW/MW/LW) is not a bad idea either. MW often gives improved response over SW.
|
|
| Back to top |
|
 |
Paul S

Joined: 20 Mar 2010
Posts: 79



|
 Posted: Jun 05, 2010 10:52 Post subject: Re: Chemical identification Posted: Jun 05, 2010 10:52 Post subject: Re: Chemical identification |
|
|
I came upon another way of testing minerals. It's rather simple; you just have to use a multi-meter. All metallic minerals (and graphite) conduct electricity very well. This can distinguish them from minerals that look like metallic minerals but are actually sulfides or oxides and don't conduct electricity very well.
You can measure the conductance by measuring the resistance of the material using the multi-meter. Conductance = 1/Resistance.
The value of the conductance will depend on the purity and the presence of fractures/disturbances in the mineral, but can give a distinction between different minerals.
|
|
| Back to top |
|
 |
Gerhard Niklasch
Joined: 27 Mar 2009
Posts: 134
Location: Munich



|
 Posted: Jun 05, 2010 16:46 Post subject: Re: Chemical identification Posted: Jun 05, 2010 16:46 Post subject: Re: Chemical identification |
|
|
| bugrock wrote: |
Having a SW and LW combined lamp works well for many specimens but sometime after purchase of that UV arrangement I picked up a MW lamp since in the fluorescent collecting sphere there are some specimens known to show better response in MW.
|
Interesting point. Come to think of it, isn't it rather remarkable that so many things respond to single emission-line wavelengths at all! (The common SWUV and LWUV sources work by filtering single lines out of the spectrum of mercury-vapor lamps; not sure about MW without looking it up.)
Unfortunately MW lamps, let alone 3-wavelength setups, are relatively expensive.
--Yet another feature that's occasionally useful is magnetism.
Let me pull some (crossed-eyes stereo) illustrations from my hat, for this and for UV as well...
| Description: |
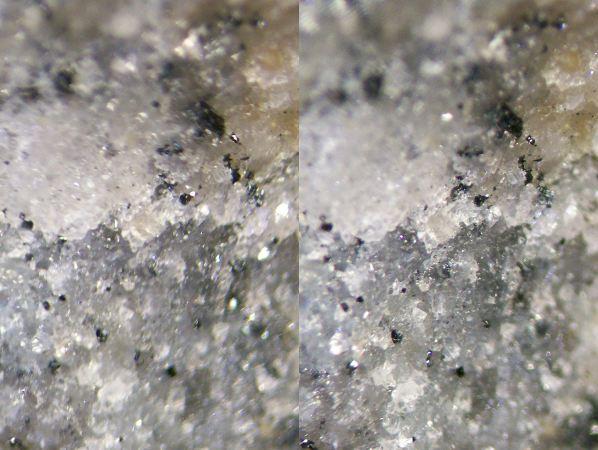
| Here's (a 12x18.4mm part of) a little piece of black-pepper-and-white-salt matrix from Långban (collection id 10SESJcx3, overall dimensions 36x30x23mm). So what's what? The light parts of the matrix are mostly carbonates (calcite and/or dolomite with some Mn); Tilasite may also be present. What about the lustrous dark grains? |
|
| Viewed: |
23863 Time(s) |

|
| Description: |
There's no lack of candidates for the dark stuff - Hausmannite, Hematite, Jacobsite, Magnetite,... and the grains are small and aren't idiomorphic. I don't have a microprobe at home, and on these scales, I'm not very good at making scratches either.
Time to walk over to the refrigerator and pick up one of those little magnets. Although it's a fairly weak one, it attaches itself to the side of the specimen with a firm clac. This rather narrows down the possibilities: Magnetite or its Mn analogue, Jacobsite. (Given what else is present on this specimen and given known assemblages according to the aforementioned book, Jacobsite is somewhat more likely.)
Field of view 8.9x12.6mm. |
|
| Viewed: |
23879 Time(s) |

|
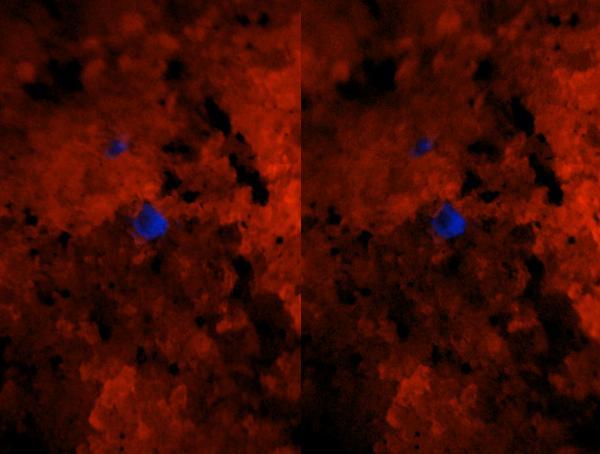
| Description: |
| The main item here is almost invisible on the first picture, and still not very impressive when we zoom in (field of view now 3.2x4.8mm, dimmed halogen bulb and white-LED fill-in). Imagine an almost colorless clear hexagonal prism about half a mm across... |
|
| Viewed: |
23892 Time(s) |
|
| Description: |
Turning off the lights and shining the SWUV lamp at it, the little Swedenborgite now stands out prominently blue against the bright orange background! Same field as preceding picture.
Now find this on the first picture! :) (It's near the center of the frame.) |
|
| Viewed: |
23887 Time(s) |
|
|
|
| Back to top |
|
 |
Ed Huskinson

Joined: 15 Apr 2009
Posts: 318
Location: Kingman, Arizona



|
 Posted: Jun 06, 2010 20:31 Post subject: Re: Chemical identification Posted: Jun 06, 2010 20:31 Post subject: Re: Chemical identification |
|
|
Oiga tocayo!!! A simple test to distinguish chrysocolla from other copper oxides (turquoise, generally) is to touch your tongue to the specimen in question. Chrysocolla sticks to your tongue. Azurite, malachite and turquoise do not. Quick and easy, and a test used by geologists throughout the southwestern US, particularly those of us who have worked in copper exploration.
Hope this helps.
Ed in Kingman
_________________
La respuesta está en las rocas!! Estudiadlas!!
Ed |
|
| Back to top |
|
 |
Luiz Menezes
Joined: 10 Dec 2009
Posts: 140
Location: Belo Horizonte


|
 Posted: Jun 06, 2010 23:05 Post subject: Re: Chemical identification Posted: Jun 06, 2010 23:05 Post subject: Re: Chemical identification |
|
|
Muchas gracias, Antônio.
Luiz
|
|
| Back to top |
|
 |
Eduardoo
Joined: 01 Jun 2010
Posts: 72
Location: Quito


|
 Posted: Jun 07, 2010 09:58 Post subject: Re: Chemical identification Posted: Jun 07, 2010 09:58 Post subject: Re: Chemical identification |
|
|
Hi Ed (tocayo).
I´ve been sucking the thing to dead because I really want to know what it is, but to no avail.
Of course we know in Spanish that “baba de mudo no pega” (Maybe Jordi, who speaks better English can help translate that).
I still thing the best candidate is Chrysocolla.
|
|
| Back to top |
|
 |
Paul S

Joined: 20 Mar 2010
Posts: 79



|
 Posted: Jun 07, 2010 10:30 Post subject: Re: Chemical identification Posted: Jun 07, 2010 10:30 Post subject: Re: Chemical identification |
|
|
| Ed, thank you very much for that interesting test you posted. I tested it on a piece of what I thought to be malachite. It turned out to be chrysocolla. I had not found an easy test yet to distinguish between closely related copper carbonates, but this one worked! I did feel a bit weird licking my specimen. I made sure no one was watching ;-)
|
|
| Back to top |
|
 |
Ed Huskinson

Joined: 15 Apr 2009
Posts: 318
Location: Kingman, Arizona



|
 Posted: Jun 07, 2010 15:24 Post subject: Re: Chemical identification Posted: Jun 07, 2010 15:24 Post subject: Re: Chemical identification |
|
|
Yeah, well, that's why we're called "rock-lickers" by the rest of society. I'm 'way past the point of caring and lick them openly, although I've taken to carrying a spray bottle in my vehicle to spray things off, and only use the licker test to distinguish chrysocolla, some clays, and some zeolites. People freak when you take a little nibble and chew it, to differentiate between claystones, mudstones, and siltstones..
Another interesting one that works on some sulphide-looking specimens (well, ore specimens) is to give the rock a sharp rap with a hammer and then quickly take a whiff. If you smell garlic, odds are that the thing contains tellurium. It's weird, but kind of neat when it works, and you've won five bucks from your disbelieving doubtful Thomas of a field assistant.because the assays came back with Te values.
Keep up the good work and send us some pictures of your other rocks.
'sta luego
_________________
La respuesta está en las rocas!! Estudiadlas!!
Ed |
|
| Back to top |
|
 |
Jason
Joined: 31 Dec 2008
Posts: 254
Location: atlanta



|
 Posted: Jun 07, 2010 23:53 Post subject: Re: Chemical identification Posted: Jun 07, 2010 23:53 Post subject: Re: Chemical identification |
|
|
| Hey ed does this licking test also work on cold metal in mid winter...hmm..:):):):)
|
|
| Back to top |
|
 |
|


