| View previous topic :: View next topic |
| Author |
Message |
Bob Morgan
Joined: 18 Jan 2018
Posts: 251
Location: Savannah, Georgia



|
 Posted: Jan 24, 2025 07:32 Post subject: Re: Limonitized garnet? Posted: Jan 24, 2025 07:32 Post subject: Re: Limonitized garnet? |
|
|
The square outline with 90 degree angles is made by 4 dodecahedral faces around the middle of the crystal as you look down on it from the top. In the first photo there are still 4 faces forming a pyramid toward the camera. The ridges that separate them are discernable. The hidden side has the other 4 faces making a pyramid to complete the twelve of the dodecahedron.
If you rotate the crystal around one of its three sided pyramids you will get the square cross section again.
As much as I love pyrite and have collected limonite pseudo's this is not one of them. It would be an awefully skewed development to have large cube faces around the sides and then have large octahedral pyramids on both ends.
|
|
| Back to top |
|
 |
Bob Carnein
Joined: 22 Aug 2013
Posts: 359
Location: Florissant, CO



|
 Posted: Jan 24, 2025 11:24 Post subject: Re: Limonitized garnet? Posted: Jan 24, 2025 11:24 Post subject: Re: Limonitized garnet? |
|
|
| OK, these certainly look like a simple rhombic dodecahedron.
|
|
| Back to top |
|
 |
Robson Vieira
Joined: 05 Jan 2017
Posts: 60
Location: São Paulo


|
 Posted: Jan 24, 2025 11:27 Post subject: Re: Limonitized garnet? Posted: Jan 24, 2025 11:27 Post subject: Re: Limonitized garnet? |
|
|
| Bob Morgan wrote: | The square outline with 90 degree angles is made by 4 dodecahedral faces around the middle of the crystal as you look down on it from the top. In the first photo there are still 4 faces forming a pyramid toward the camera. The ridges that separate them are discernable. The hidden side has the other 4 faces making a pyramid to complete the twelve of the dodecahedron.
If you rotate the crystal around one of its three sided pyramids you will get the square cross section again.
As much as I love pyrite and have collected limonite pseudo's this is not one of them. It would be an awefully skewed development to have large cube faces around the sides and then have large octahedral pyramids on both ends. |
Yeah Bob, you have described properly the crystal, the hidden side as well. It leads me to believe it is a garnet so. Thank your attention.
|
|
| Back to top |
|
 |
Robson Vieira
Joined: 05 Jan 2017
Posts: 60
Location: São Paulo


|
 Posted: Jan 24, 2025 11:29 Post subject: Re: Limonitized garnet? Posted: Jan 24, 2025 11:29 Post subject: Re: Limonitized garnet? |
|
|
| Bob Carnein wrote: | | OK, these certainly look like a simple rhombic dodecahedron. |
It was my very first impression. I think it is not a shame to call it a garnet.
|
|
| Back to top |
|
 |
Roger Warin

Joined: 23 Jan 2013
Posts: 1246



|
 Posted: Jan 24, 2025 16:26 Post subject: Re: Limonitized garnet? Posted: Jan 24, 2025 16:26 Post subject: Re: Limonitized garnet? |
|
|
Hello,
I believe in a pseudomorphosis of a garnet.
The texture of this specimen is not that of a crystal.
I think we often forget that water is an extracting agent of SiO2.
Obviously the water must be in a "critical" state (high temperatures and pressures).
SiO2 is then soluble in water to give various silicic and polysilicic acids. One or more iron oxides remain in the mixture.
These words are only a suggestion.
Roger.
|
|
| Back to top |
|
 |
Herwig
Joined: 04 Jan 2016
Posts: 43
Location: Hasselt


|
 Posted: Jan 24, 2025 21:53 Post subject: Re: Limonitized garnet? Posted: Jan 24, 2025 21:53 Post subject: Re: Limonitized garnet? |
|
|
Thus:
a garnet crystal, having the (classic) shape of a rhombic dodecahedron, has been replaced by a mixture of iron oxides & hydroxides, thereby retaining the shape of the original crystal. Since we don't know the actual composition of the thing that replaced the garnet, but we assume it to contain oxides and also probably hydroxides of iron, it's best to call that material "limonite", especially since it looks "earthy".
more info on limonite here: https://www.mindat.org/min-2402.html
in short:
limonite pseudomorph after garnet
Cheers, Herwig
|
|
| Back to top |
|
 |
Robson Vieira
Joined: 05 Jan 2017
Posts: 60
Location: São Paulo


|
 Posted: Jan 26, 2025 08:49 Post subject: Re: Limonitized garnet? Posted: Jan 26, 2025 08:49 Post subject: Re: Limonitized garnet? |
|
|
| Roger Warin wrote: | Hello,
I believe in a pseudomorphosis of a garnet.
The texture of this specimen is not that of a crystal.
I think we often forget that water is an extracting agent of SiO2.
Obviously the water must be in a "critical" state (high temperatures and pressures).
SiO2 is then soluble in water to give various silicic and polysilicic acids. One or more iron oxides remain in the mixture.
These words are only a suggestion.
Roger. |
Hi friend, i really enjoy to know that. Great explanation. Thanks
|
|
| Back to top |
|
 |
Robson Vieira
Joined: 05 Jan 2017
Posts: 60
Location: São Paulo


|
 Posted: Jan 26, 2025 08:54 Post subject: Re: Limonitized garnet? Posted: Jan 26, 2025 08:54 Post subject: Re: Limonitized garnet? |
|
|
| Herwig wrote: | Thus:
a garnet crystal, having the (classic) shape of a rhombic dodecahedron, has been replaced by a mixture of iron oxides & hydroxides, thereby retaining the shape of the original crystal. Since we don't know the actual composition of the thing that replaced the garnet, but we assume it to contain oxides and also probably hydroxides of iron, it's best to call that material "limonite", especially since it looks "earthy".
more info on limonite here: https://www.mindat.org/min-2402.html
in short:
limonite pseudomorph after garnet
Cheers, Herwig |
Hi friend. Interesting topic you stated. The link you provided says that 'limonite' is goethite at most, but can be formed by lepidocrosite, hematite...as well. I'm going to do as you said, call it as limonite pseudo after garnet, which sounds to me pretty nice. Thank you so much.
|
|
| Back to top |
|
 |
Robson Vieira
Joined: 05 Jan 2017
Posts: 60
Location: São Paulo


|
 Posted: Jan 26, 2025 09:00 Post subject: Re: Limonitized garnet? Posted: Jan 26, 2025 09:00 Post subject: Re: Limonitized garnet? |
|
|
I'm very grateful for the help you all provided to me, especially in terms of clearance your points over the topic that allowed me to identify and better yet, understand related questions about environment, mineral formation, pseudomorphism, wearing conditions and so on.
Thank you all
|
|
| Back to top |
|
 |
Roger Warin

Joined: 23 Jan 2013
Posts: 1246



|
 Posted: Jan 26, 2025 15:43 Post subject: Re: Limonitized garnet? Posted: Jan 26, 2025 15:43 Post subject: Re: Limonitized garnet? |
|
|
| Isn't IMA, limonite, a rock and not a mineral species?
|
|
| Back to top |
|
 |
alfredo
Site Admin

Joined: 30 Jan 2008
Posts: 1014



|
 Posted: Jan 26, 2025 18:20 Post subject: Re: Limonitized garnet? Posted: Jan 26, 2025 18:20 Post subject: Re: Limonitized garnet? |
|
|
| "Limonite" is a field term for iron oxides which have not been analyzed and for which the species is not known, although 90% of the time it is either goethite or a mixture of hematite and goethite. So, its usage is similar to field terms like "psilomelane" (unanalyzed hard manganese oxides) or "wad" (unanalyzed soft manganese oxides).
|
|
| Back to top |
|
 |
Roger Warin

Joined: 23 Jan 2013
Posts: 1246



|
 Posted: Jan 26, 2025 23:17 Post subject: Re: Limonitized garnet? Posted: Jan 26, 2025 23:17 Post subject: Re: Limonitized garnet? |
|
|
Thank you, Alfredo,
My comment concerns the IMA, whose scientific rigor, obviously necessary, sometimes bewilders the amateur. Scientists obviously have a background.
It seems to me that there is a difference in philosophy between mineralogists and gemologists, who sometimes seem more didactic. I'm not talking about “meteorists”, for whom olivine does exist.
My comment concerns not the scientific articles, but the contents of Fleischer's Glossary. This catalog is widely used by amateurs as a reference. It even encourages amateurs to collect systematically, since it includes a checkbox.
However, the Glossary does not mention olivine. I think it's even banned from publication... except when it talks about the olivine group.
What's the olivine group? Need we remind you that it's one of the main components of the Earth's mantle?
This problem is of course due to the existence of solid solutions. This definition (which I'm not criticizing) is bizarre, since there is no solvent.
My wish is that the Glossary will insert a few sheets to remind us of the historical existence of certain ubiquitous solid solutions, whereas limit poles are almost an illusion, it seems to me. There are even cases where the second limit does not exist.
I'm probably a utopian.
|
|
| Back to top |
|
 |
alfredo
Site Admin

Joined: 30 Jan 2008
Posts: 1014



|
 Posted: Jan 27, 2025 00:31 Post subject: Re: Limonitized garnet? Posted: Jan 27, 2025 00:31 Post subject: Re: Limonitized garnet? |
|
|
| Roger, I don't think you can insist that Fleischer's glossary should include more than what it states to be its intent: a list of species. So anything that is not a "species" won't be included. If you want a list of species + groups + isomorphous series + historical names, then you yourself should publish "Warin's Glossary of Species, Series, Groups, and Historical Names". (And yes, I will buy it ;))
|
|
| Back to top |
|
 |
Herwig
Joined: 04 Jan 2016
Posts: 43
Location: Hasselt


|
 Posted: Jan 27, 2025 03:56 Post subject: Re: Limonitized garnet? Posted: Jan 27, 2025 03:56 Post subject: Re: Limonitized garnet? |
|
|
| alfredo wrote: | | Roger, I don't think you can insist that Fleischer's glossary should include more than what it states to be its intent: a list of species. So anything that is not a "species" won't be included. If you want a list of species + groups + isomorphous series + historical names, then you yourself should publish "Warin's Glossary of Species, Series, Groups, and Historical Names". (And yes, I will buy it ;)) |
I'm afraid it will be more of an encyclopedia, than a book. :-)
IMO more work should be done on Mindat, to make it THE place to look for ALL names of species, groups, series and historical names.
And to clearly state in the About section of every "mineral page", what that page is for (a species, a series, a group), as well as what the possible "connections" with other pages are.
An example: on the forsterite and the fayalite pages, it should be mentioned they are the end members of a continuous series, and intermediate compositions have been called olivine, but that name is no longer a species name.
Right now, the forsterite page only has two lines of text:
| mindat wrote: | Olivine Group. Fayalite-Forsterite Series, and the Forsterite-Tephroite Series.
The magnesium analogue of Fayalite, Tephroite, and Calcio-Olivine. |
So, there is still a lot of work to be done.
Hopefully one day we'll get there. :-)
|
|
| Back to top |
|
 |
Roger Warin

Joined: 23 Jan 2013
Posts: 1246



|
 Posted: Jan 27, 2025 17:12 Post subject: Re: Limonitized garnet? Posted: Jan 27, 2025 17:12 Post subject: Re: Limonitized garnet? |
|
|
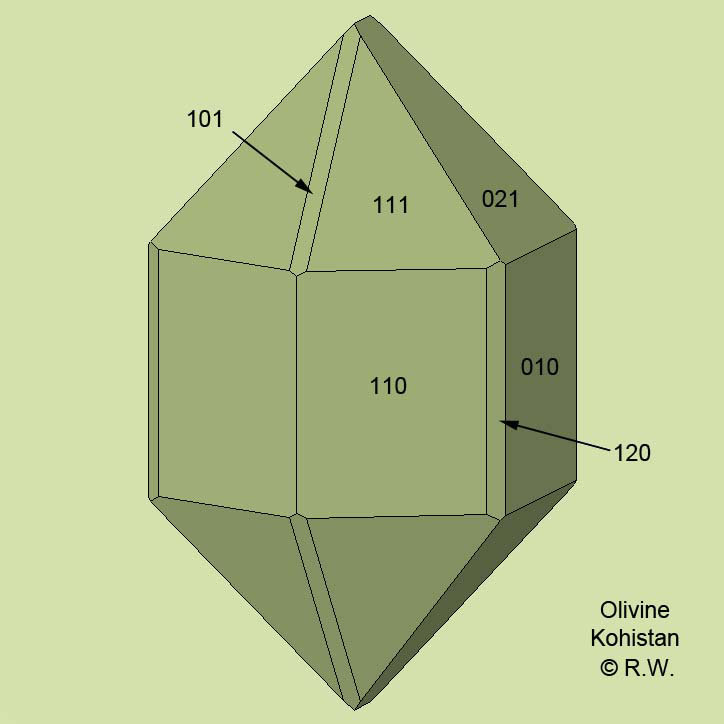
Please help me.
After 70 years of collecting, I no longer know how to write a label for this automorphic mineral, whose face indices I have noted.
I think I've reached a good level of mineralogical knowledge, even though I'm still an amateur.
At the moment, I'm inclined to use the old name “peridot”, which might escape controversy.
What would a museum curator do?
| Mineral: | unknown |
| Description: |
|
| Viewed: |
2752 Time(s) |

|
| Mineral: | peridot model |
| Description: |
|
| Viewed: |
2770 Time(s) |
|
|
|
| Back to top |
|
 |
|





