| View previous topic :: View next topic |
| Author |
Message |
Duncan Miller

Joined: 25 Apr 2009
Posts: 138
Location: South Africa



|
 Posted: Aug 23, 2011 04:34 Post subject: Is this a twin? Posted: Aug 23, 2011 04:34 Post subject: Is this a twin? |
|
|
Is this a twin, or just a fortuitous etch feature in beryl? I will post photos of two different orientations to show the re-entrants.
Duncan
| Description: |
Beryl
Brazil (reportedly)
40 mm long |
|
| Viewed: |
83552 Time(s) |

|
| Description: |
Beryl
Brazil (reportedly)
40 mm long |
|
| Viewed: |
83597 Time(s) |

|
|
|
| Back to top |
|
 |
Pete Richards
Site Admin

Joined: 29 Dec 2008
Posts: 846
Location: Northeast Ohio



|
 Posted: Aug 23, 2011 07:49 Post subject: Re: Is this a twin? Posted: Aug 23, 2011 07:49 Post subject: Re: Is this a twin? |
|
|
I believe this is not a twin. Twinning is unknown in beryl. One principle of the identification of twins is that they should occur too frequently to be explainable by chance, and one lone occurrence doesn't qualify.
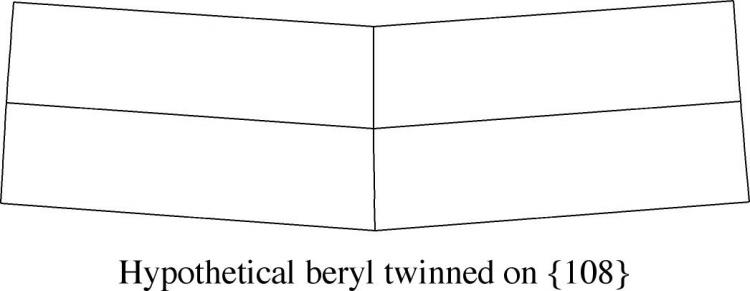
That said, this specimen certainly seems to have a "kink" in it, and its symmetric morphology is suggestive of a twin. However, the shallow angle of deviation between the two halves is less than one would normally expect from a twin, and modeling using a crystal drawing program indicates that the twin law would have quite high indices - approximately (108), which is also a strike against it.
My guess is that this crystal was broken and re-healed slightly out of line, perhaps early in its growth, and that the dark line around the center represents a healing surface rather than a twin plane.
| Description: |
|
| Viewed: |
83569 Time(s) |
|
_________________
Collecting and studying crystals with interesting habits, twinning, and epitaxy |
|
| Back to top |
|
 |
Peter Megaw
Site Admin

Joined: 13 Jan 2007
Posts: 973
Location: Tucson, Arizona



|
 Posted: Aug 23, 2011 08:23 Post subject: Re: Is this a twin? Posted: Aug 23, 2011 08:23 Post subject: Re: Is this a twin? |
|
|
I agree with Pete that it was probably broken and rehealed but I might suggest you check the join carefully for glue the healing might not be that old
_________________
Siempre Adelante! |
|
| Back to top |
|
 |
John S. White
Site Admin

Joined: 04 Sep 2006
Posts: 1298
Location: Stewartstown, Pennsylvania, USA



|
 Posted: Aug 23, 2011 08:46 Post subject: Re: Is this a twin? Posted: Aug 23, 2011 08:46 Post subject: Re: Is this a twin? |
|
|
I almost made the same statement that Pete Richards made but checked first and discovered to my surprise that there is a rare twin law for beryl, {hkil} with a bar over the i. Pete would be better able to figure out what this angle should be than I.
I feel quite certain that the "healing surface" is perfectly natural.
_________________
John S. White
aka Rondinaire |
|
| Back to top |
|
 |
Duncan Miller

Joined: 25 Apr 2009
Posts: 138
Location: South Africa



|
 Posted: Aug 23, 2011 11:19 Post subject: Re: Is this a twin? Posted: Aug 23, 2011 11:19 Post subject: Re: Is this a twin? |
|
|
Thanks for the several, and swift, replies. There is no sign of glue, so I think the join is natural. It is also a bit irregular, which supports the broken-regrown explanation. If it were a twin, as Pete pointed out, the twin plane would have very high indices. Deer, Howie and Zussman 1966 give beryl twinning as "Rare; on {31bar41}, {11bar20} and {40bar41}?". What puzzled me was the symmetrical etching either side of the join, creating the re-entrant angles, but I suppose etching could have been initiated at the surface expression of the join and progressed symmetrically outwards from there.
Duncan
|
|
| Back to top |
|
 |
Pete Richards
Site Admin

Joined: 29 Dec 2008
Posts: 846
Location: Northeast Ohio



|
 Posted: Aug 23, 2011 11:52 Post subject: Re: Is this a twin? Posted: Aug 23, 2011 11:52 Post subject: Re: Is this a twin? |
|
|
John White correctly pointed out that I missed some reported twin laws for beryl. Duncan (xenolithos) mentions three twin laws from Deer Howie and Zussman; these three are echoed in Dana's System 8th edition, listing the first two as rare and the last as doubtful.
John's mention of twinning on {hkil} apparently comes from the Handbook of Mineralogy. This is a singularly useless description (not John's fault!) because h, k, and l can represent any number (and i is always equal to h+k or -(h+k), depending on whether it is written with a superior bar or not).
(There is a growing tendency to omit the i index from Miller indices in the hexagonal system, because it is superfluous, and I do so below and did so in my first post but failed to mention it).
Of the three laws Duncan quotes, {110} is highly questionable since that plane is a symmetry element for beryl, and therefore twinning on that plane does nothing to change the structure or the morphology, which is what the concept of twinning is all about. This twin law only makes sense if some special structural modification (such as ordered impurities) reduces the symmetry; in any case if the law is known for such situations, it would not be visible in the external morphology.
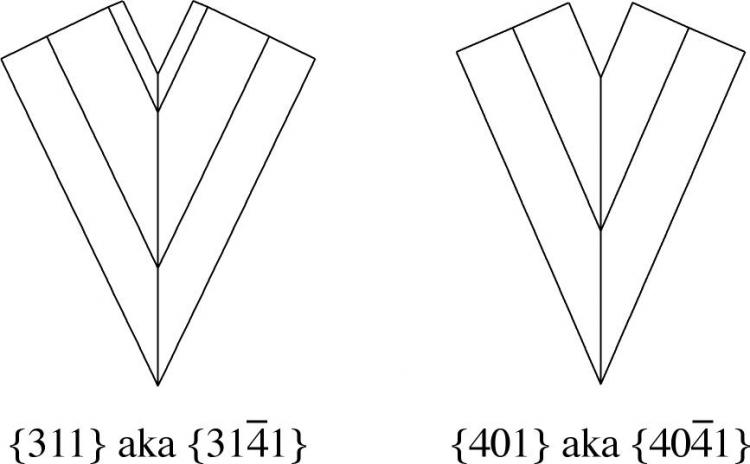
So we are left (in my mind) with there being two possible twin laws, {311} and {401}, of which the first is deemed rare and the second doubtful by the authors of Dana 8. Until I see a convincing example, I'm dubious for sure!
In any case, both of those twin laws lead to a V-shaped morphology that looks nothing like the one that started this post, as the drawing below shows.
| Description: |
|
| Viewed: |
83436 Time(s) |
|
_________________
Collecting and studying crystals with interesting habits, twinning, and epitaxy |
|
| Back to top |
|
 |
Duncan Miller

Joined: 25 Apr 2009
Posts: 138
Location: South Africa



|
 Posted: Aug 23, 2011 12:54 Post subject: Re: Is this a twin? Posted: Aug 23, 2011 12:54 Post subject: Re: Is this a twin? |
|
|
Thanks for the drawings Pete, and the clarification about the abbreviated hexagonal indices, which I had forgotten. Clearly my specimen is not twinned according to any suspected twin law for beryl. I think this clears it up then. It's not a twin, but a fortuitously etched intergrowth. It irks me when people refer to merely intergrown crystals as 'twinned' and I would not want to be guilty of that.
Duncan
|
|
| Back to top |
|
 |
PMH
Joined: 19 Jan 2012
Posts: 5


|
 Posted: Jan 19, 2012 12:47 Post subject: Re: Is this a twin? Posted: Jan 19, 2012 12:47 Post subject: Re: Is this a twin? |
|
|
I was just looking around researching twins a little, and came across this..
https://www.tulane.edu/~sanelson/eens211/twinning.htm
(link normalized by FMF)
..which looks an awful lot like the aqua of this thread -- to me, anyway.
...and the text seems to be saying that there's 5 or so types of twinning that are common in hexagonal minerals.
...so that'd mean (?) that if beryl doesn't share them, there must be some reason.
Do we know any reason for why beryl doesn't twin?
|
|
| Back to top |
|
 |
Pete Richards
Site Admin

Joined: 29 Dec 2008
Posts: 846
Location: Northeast Ohio



|
 Posted: Jan 19, 2012 14:39 Post subject: Re: Is this a twin? Posted: Jan 19, 2012 14:39 Post subject: Re: Is this a twin? |
|
|
The answers to your questions are quite complex.... For starters, note that the examples of twinning in the hexagonal class are examples from the trigonal subclass - the minerals with three-fold symmetry rather than six-fold symmetry. These minerals have fewer symmetry elements, so there are more possible planes or axes to serve as twin elements. Calcite twins commonly on the "basal plane" {001}, the plane perpendicular to the c- or vertical axis. Beryl cannot do so because that direction is already a symmetry element for beryl, and as the document says twin planes must be planes that are not already symmetry planes in the untwinned crystal.
But beyond that, the structure of some minerals is more disposed to form twins than that of other minerals. There's a substantial literature from the early 20th century (much in French or German) that discusses conditions right for formation of twins. A simplification is that if a mineral has a structure such that adding a twinning element will create an atomic configuration across that twin plane that is almost like it would be in the untwinned crystal, twinning is likely on that plane. In addition there's the general crystallographic principle that important planes (faces or twin planes) typically have simple, small Miller indices (say, all chosen from 0, 1, 2, and maybe 3), so the number of possibilities is not great. Some minerals are good candidates and some are not.
An orthorhombic example: aragonite, witherite, and cerussite all form twins on {110}, often cyclic, forming pseudohexagonal "prisms" or "bipyramids" (and more complex intergrowths). Celestite and Baryte, on the other hand, are generally considered not to form twins. These minerals all belong to the same symmetry system and class.
_________________
Collecting and studying crystals with interesting habits, twinning, and epitaxy |
|
| Back to top |
|
 |
PMH
Joined: 19 Jan 2012
Posts: 5


|
 Posted: Jan 20, 2012 10:02 Post subject: Re: Is this a twin? Posted: Jan 20, 2012 10:02 Post subject: Re: Is this a twin? |
|
|
Very cool! thanks
I never learned - or heard of (?) - that twinning always increases symmetry.
(& never managed to get classes, point groups , etc. into my head)
(& I'm a mathematician!)
(I even marvel that anyone has managed to)
..so beryl is already as symmetric as it could possibly be?
(galleries com says "The highest symmetry class of the hexagonal system is the Dihexagonal Dipyramidal Class.")
If so, isn't that enough to prove that beryl has no twinning?
Do the alleged twinning possibilities for beryl increase its symmetry?
|
|
| Back to top |
|
 |
Pete Richards
Site Admin

Joined: 29 Dec 2008
Posts: 846
Location: Northeast Ohio



|
 Posted: Jan 20, 2012 11:42 Post subject: Re: Is this a twin? Posted: Jan 20, 2012 11:42 Post subject: Re: Is this a twin? |
|
|
| PMH wrote: | Very cool! thanks
I never learned - or heard of (?) - that twinning always increases symmetry.
[snip]
..so beryl is already as symmetric as it could possibly be?
(galleries com says "The highest symmetry class of the hexagonal system is the Dihexagonal Dipyramidal Class.")
If so, isn't that enough to prove that beryl has no twinning?
Do the alleged twinning possibilities for beryl increase its symmetry? |
It's not really the case that twinning increases symmetry. When we speak of symmetry, we're speaking of the symmetry of the atomic arrangement within the crystal structure, which is the same at any equivalent position in the structure. The twin plane is only present at the interface between the two twinned individual, therefore it is not part of the symmetry of the individual.
Western practice does not generally discuss the overall symmetry of the twinned aggregate (contrary to Russian practice, and least among some). However, the overall symmetry of a twin is often lower than the symmetry of the individual. The hypothetical twins posted earlier would have an overall symmetry with two mirror planes, one of them the twin plane. The individual beryl has a 6-fold rotational axis, two non-equivalent sets of 6 two-fold rotational axes, and mirror planes perpendicular to all axes. LOTS more symmetry!
Given that beryl is hexagonal, yes, it has the highest possible amount of symmetry. But that does not prove it could not be twinned, since twinning does not increase the symmetry of the crystal.
_________________
Collecting and studying crystals with interesting habits, twinning, and epitaxy |
|
| Back to top |
|
 |
PMH
Joined: 19 Jan 2012
Posts: 5


|
 Posted: Jan 20, 2012 14:02 Post subject: Re: Is this a twin? Posted: Jan 20, 2012 14:02 Post subject: Re: Is this a twin? |
|
|
Thanks again!
You're obviously right about the xl of this thread.
...something that I should have noticed immediately myself
I guess I must've taken the Page that I saw this on as truth - w/o analyzing it for myself.
...but OTOH, it was part of a course at a university.
(tulane edu ~sanelson eens211 twinning htm)
He says:
o "What happens is that lattice points in one crystal are shared as lattice points in another crystal adding apparent symmetry to the crystal pairs. Twinning, because it adds symmetry, never occurs in relation to the existing symmetry of the crystal."
I guess this is the RUssian usage.
o "If the twin law can be defined by a simple planar composition surface, the twin plane is always parallel to a possible crystal face and never parallel to an existing plane of symmetry (remember that twinning adds symmetry). "
o "[111] - The twin axis perpendicular to an octahedral face adds three fold rotational symmetry. "
That seems clearly wrong, by visual examination of his example.
o "Iron Cross [001] - The mineral pyrite (FeS2) often shows the iron cross made of the interpenetration of two pyritohedrons. Since this occurs in the class 2/m, with no 4-fold rotation axes, the [001] twin axis gives the mineral apparent 4-fold symmetry about 3 perpendicular axes. "
That seems right.
...so I can't figure out what he's thinking..
(At first, I thought that the problem was just his use of "add", but then it seems like in the examples he gives, he's clearly talking about adding symmetry elements.)
..can you?
|
|
| Back to top |
|
 |
PMH
Joined: 19 Jan 2012
Posts: 5


|
 Posted: Jan 20, 2012 14:08 Post subject: Re: Is this a twin? Posted: Jan 20, 2012 14:08 Post subject: Re: Is this a twin? |
|
|
(I got into this when I was trying to understand why two (adjacent) (prism) faces of an amazonite xl showed "reentrant" angles, which I've taken to imply (suggest?) twinning.
...although it seems clear now that that's not twinning.
Not to pollute this thread with a branching issue, but what's going on here?
Is it just a parallel-growth thing?..
..which I'm familiar with in quartz
...which would be just renewed growth of the xl..
..possibly under different conditions
(which would cause it to look like one xl was growing partly to the side of, partly overlapping, the original)
|
|
| Back to top |
|
 |
Pete Richards
Site Admin

Joined: 29 Dec 2008
Posts: 846
Location: Northeast Ohio



|
 Posted: Jan 20, 2012 14:36 Post subject: Re: Is this a twin? Posted: Jan 20, 2012 14:36 Post subject: Re: Is this a twin? |
|
|
| PMH wrote: | Thanks again!
You're obviously right about the xl of this thread.
...something that I should have noticed immediately myself
I guess I must've taken the Page that I saw this on as truth - w/o analyzing it for myself.
...but OTOH, it was part of a course at a university.
(tulane edu ~sanelson eens211 twinning htm)
He says:
o "What happens is that lattice points in one crystal are shared as lattice points in another crystal adding apparent symmetry to the crystal pairs. Twinning, because it adds symmetry, never occurs in relation to the existing symmetry of the crystal."
I guess this is the RUssian usage.
o "If the twin law can be defined by a simple planar composition surface, the twin plane is always parallel to a possible crystal face and never parallel to an existing plane of symmetry (remember that twinning adds symmetry). "
o "[111] - The twin axis perpendicular to an octahedral face adds three fold rotational symmetry. "
That seems clearly wrong, by visual examination of his example.
o "Iron Cross [001] - The mineral pyrite (FeS2) often shows the iron cross made of the interpenetration of two pyritohedrons. Since this occurs in the class 2/m, with no 4-fold rotation axes, the [001] twin axis gives the mineral apparent 4-fold symmetry about 3 perpendicular axes. "
That seems right.
...so I can't figure out what he's thinking..
(At first, I thought that the problem was just his use of "add", but then it seems like in the examples he gives, he's clearly talking about adding symmetry elements.)
..can you? |
I think this is largely a question of semantics. I think it is reasonable to think of adding a symmetry element (a mirror plane, for example) to a crystal to twin it. But the added symmetry element characterizes the twin only - and in fact the twin boundary only (though things are trickier in the case of penetration twins) - it does not change the symmetry of the individuals that comprise the twin. Most crystallograpers do not speak of adding a symmetry element, but rather of "twinning by reflection on the plane (hkll)" or "twinning by rotation about the axis [uvw]".
A twin operation cannot be part of the symmetry set for the untwinned mineral, because that twin operation would not change the orientation of the lattice or crystal structure.
"[111]"... You are right. The penetration twin of cubes shown is achieved by a two-fold rotation about [111], not three-fold. A three-fold rotation (120°) maps the cube onto itself.
"Iron cross"... The description is OK. It would require a rotation of 90° about a [100] axis. It can also be described as a penetration twin by reflection across the plane (110) or by 180° rotation about the axis [110]. Generally, crystallographers frown on twins by rotation about an angle other than 180° (I am reluctant to claim that such twins do not exist...), and usually an alternative specification can be found, as here. The professor speaks of "apparent" four-fold symmetry, and indeed it is only apparent.
_________________
Collecting and studying crystals with interesting habits, twinning, and epitaxy |
|
| Back to top |
|
 |
PMH
Joined: 19 Jan 2012
Posts: 5


|
 Posted: Jan 20, 2012 14:49 Post subject: Re: Is this a twin? Posted: Jan 20, 2012 14:49 Post subject: Re: Is this a twin? |
|
|
| Thanks again.
|
|
| Back to top |
|
 |
|




