| View previous topic :: View next topic |
| Author |
Message |
RayStraw
Joined: 21 Jul 2018
Posts: 47
Location: Bloomington Illinois


|
 Posted: Jun 04, 2019 09:39 Post subject: Stability of Azurite vs Malachite Posted: Jun 04, 2019 09:39 Post subject: Stability of Azurite vs Malachite |
|
|
The reaction of azurite to malachite requires the addition of water and the subsequent removal of carbon dioxide. The volume of two azurite molecules (with three copper atoms per molecule) to yield three malachite molecules (with two copper atoms per molecule) results in 9.5% porosity. This porosity should permit the introduction of water and the removal of carbon dioxide from the malachite-azurite interface.
Can anyone provide any documentation of azurite vs malachite stability from criteria such as thermodynamic or otherwise?
|
|
| Back to top |
|
 |
Robert Seitz
Joined: 29 Dec 2015
Posts: 261
Location: Phoenix, AZ



|
 Posted: Jun 04, 2019 09:46 Post subject: Re: Stability of Azurite vs Malachite Posted: Jun 04, 2019 09:46 Post subject: Re: Stability of Azurite vs Malachite |
|
|
Go on Google and search: "stability relations of malachite and azurite"
An article by B.W. Vink, MINERALOGICAL MAGAZINE, MARCH 1986, VOL. 50, PP. 41-7, will show.
|
|
| Back to top |
|
 |
RayStraw
Joined: 21 Jul 2018
Posts: 47
Location: Bloomington Illinois


|
 Posted: Jun 06, 2019 10:37 Post subject: Re: Stability of Azurite vs Malachite Posted: Jun 06, 2019 10:37 Post subject: Re: Stability of Azurite vs Malachite |
|
|
| Robert Seitz wrote: | Go on Google and search: "stability relations of malachite and azurite"
An article by B.W. Vink, MINERALOGICAL MAGAZINE, MARCH 1986, VOL. 50, PP. 41-7, will show. |
The paper does a good job of explaining the formation of azurite and/or malachite.
What I'm interested in is whether malachite can pseudomorph to azurite. The are lots of malachite after azurite pseudos. Can the lack of azurite after malachite pseudos be explained by chemical/thermodynamic arguments?
Thank you.
Ray Straw
|
|
| Back to top |
|
 |
lluis
Joined: 17 Nov 2006
Posts: 719


|
 Posted: Jun 06, 2019 15:41 Post subject: Re: Stability of Azurite vs Malachite Posted: Jun 06, 2019 15:41 Post subject: Re: Stability of Azurite vs Malachite |
|
|
Hi, Ray
The thermodynamical explanation for transformation from azurite to malachite is just that malachite is the stable item in Earth atmosphere.
Azurite is stable at pressures of around 5 (or higher!) atmospheres CO2, which is not the case in Earth surface (around an atmosphere, in total (and far less in CO2), so.....
All tends to be in its more stable state, so....
Hope it serves
Lluís
|
|
| Back to top |
|
 |
John Betts
Joined: 07 Jun 2012
Posts: 210
Location: New York City


|
 Posted: Jun 06, 2019 17:37 Post subject: Re: Stability of Azurite vs Malachite Posted: Jun 06, 2019 17:37 Post subject: Re: Stability of Azurite vs Malachite |
|
|
Ray, the key to pseudomorphism, as you wrote, is that the primary mineral is a larger volume than the secondary mineral. Malachite is less volume than same weight of azurite, resulting in porous pseudomorphs.
Reversing the process, even if it were possible, would result in a larger volume of azurite replacing primary malachite, which would cause the specimen to "rupture" destroying the possible pseudomorph.
_________________
John Betts |
|
| Back to top |
|
 |
Peter Lemkin
Joined: 18 Nov 2016
Posts: 403
Location: Prague


|
 Posted: Jun 07, 2019 01:25 Post subject: Re: Stability of Azurite vs Malachite Posted: Jun 07, 2019 01:25 Post subject: Re: Stability of Azurite vs Malachite |
|
|
| John Betts wrote: | Ray, the key to pseudomorphism, as you wrote, is that the primary mineral is a larger volume than the secondary mineral. Malachite is less volume than same weight of azurite, resulting in porous pseudomorphs.
Reversing the process, even if it were possible, would result in a larger volume of azurite replacing primary malachite, which would cause the specimen to "rupture" destroying the possible pseudomorph. |
I thought it was chemo-thermodynamics that drove changes from one mineral to another. I'm confused how volume would play into that unless pressure generated in a closed space would change the thermodynamic direction. I've had lots of chemistry and physics, but did not earn my graduate degree in either. Most pseudomorphs take up the same space as the original mineral, but changed due to changing hydration, chemistry, or other physical/thermodynamic factors - and it seems logical to me [though I may be wrong] that give the proper conditions the reaction could be reversible or yet go a third way. I collect and enjoy pseudomorphs, but don't claim to know all there is to know about what drives them...just throwing out the question to any experts in the 'house'.
|
|
| Back to top |
|
 |
lluis
Joined: 17 Nov 2006
Posts: 719


|
 Posted: Jun 07, 2019 04:06 Post subject: Re: Stability of Azurite vs Malachite Posted: Jun 07, 2019 04:06 Post subject: Re: Stability of Azurite vs Malachite |
|
|
Hi, Peter, List
Azurite converts to malachite at atmospheric pressure, in presence of humidity.
But if you get some malachite and place it in a closed container/reactor where pressure of CO2 is over the limit at which azurite is stable, , and manage to maintain enough time, you would return to have azurite after malachite. In case malachite used was a malachite after azurite, then, probably you would get again the crystal of azurite in same shape, but being many minicrystals in front of the bigger ones of the original azurite. And those, probably would not be very stable to touch, so, even occupying same space as original, resistance to touch would not be that great....
In case you would have again azurite, same reactor, high CO2 pressure and water and heat to make an hydrothermal anything (reaction by sure; name: pseudomorphing?...) to get azurite, in probably any other shape...
With best wishes
Lluís
|
|
| Back to top |
|
 |
Roger Warin

Joined: 23 Jan 2013
Posts: 1250



|
 Posted: Jun 07, 2019 08:39 Post subject: Re: Stability of Azurite vs Malachite Posted: Jun 07, 2019 08:39 Post subject: Re: Stability of Azurite vs Malachite |
|
|
Hi,
I think the answer to this question has already been provided by Vink (1986), as Robert Seitz recalls.
The crystalline structures are very different and it is a chemical process that has occurred. This process is a precipitation from basin fluids. Here there is neither hydrothermal nor alteration.
The decrease in molecular volume (as recalled by John Betts) allows the subsequent slow and unbroken reaction of the parent crystal (azurite). This chemical reaction depends solely on the partial pressure of CO2.
A tropical climate seems favorable (temperature).
Cold geographical zones may slow down the phenomenon of pseudomorphosis (?). It seems to me that in Belgium we do not find peudomorphoses of azurite. But azurite crystals are rarer than true malachite crystals in Belgium.
Microcavities result from lack of material. The bicarbonate anions remove Cu2+ cupric cations by solubility.
It is for this reason that in Nature the reaction is irreversible.
Azurite Unit cell Vol = 301.97 A3; Z = 2.
2 units = 603.94
Malachite Unit cell Volume = 364.35 Å3 Z = 4.
Azurite: Cu3(CO3)2 (OH)2
Malachite: Cu2 (CO3) (OH) 2 - [Cu2+ + CO32-] formally.
The dependence of the CO2 partial pressure is obvious. It is conditioned by the pH and temperature.
Roger.
|
|
| Back to top |
|
 |
RayStraw
Joined: 21 Jul 2018
Posts: 47
Location: Bloomington Illinois


|
 Posted: Jun 07, 2019 12:34 Post subject: Re: Stability of Azurite vs Malachite Posted: Jun 07, 2019 12:34 Post subject: Re: Stability of Azurite vs Malachite |
|
|
Thank you everyone for your insights.
I will consider these thoughts in my forthcoming paper of azurite to malachite pseudomorphs.
Best regards,
Ray Straw
|
|
| Back to top |
|
 |
Dehne
Joined: 27 Mar 2013
Posts: 11
Location: Hobart, Tasmania


|
 Posted: Jun 07, 2019 17:23 Post subject: Re: Stability of Azurite vs Malachite Posted: Jun 07, 2019 17:23 Post subject: Re: Stability of Azurite vs Malachite |
|
|
I am mining a significant deposit of azurite that sits well above the water table in a kalonite lens and is affected by groundwater. Very little of the azurite has altered to malachite. Deep time evolutionary age for the deposit is -minimum 300my and oldest 500my. Research paper in 2017 advised it is a primary diagenetic azurite deposit arising from deep seated high pressure fluids injected into an altered kaolin matrix. Ie the field data dictates the genesis, not the formation age.
It's been remarked that why has the azurite not all been altered to malachite?
Needs another paper on this enigma. This discussion has helped moved that along.
_________________
Dehne McLaughlin
Hobart
Tasmania
Australia |
|
| Back to top |
|
 |
Peter Megaw
Site Admin

Joined: 13 Jan 2007
Posts: 975
Location: Tucson, Arizona



|
 Posted: Jun 07, 2019 17:24 Post subject: Re: Stability of Azurite vs Malachite Posted: Jun 07, 2019 17:24 Post subject: Re: Stability of Azurite vs Malachite |
|
|
This is a most interesting thread....that stimulates a few comments.
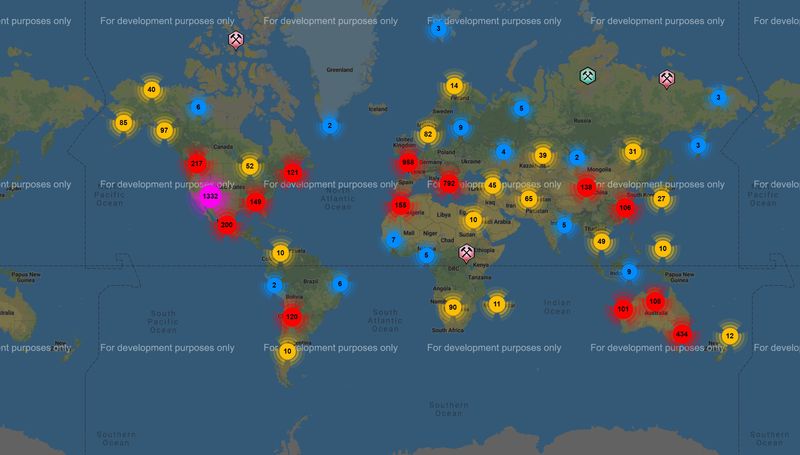
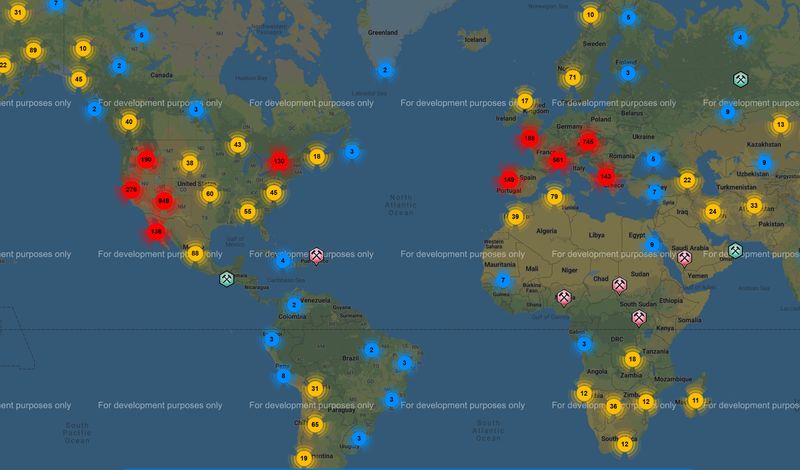
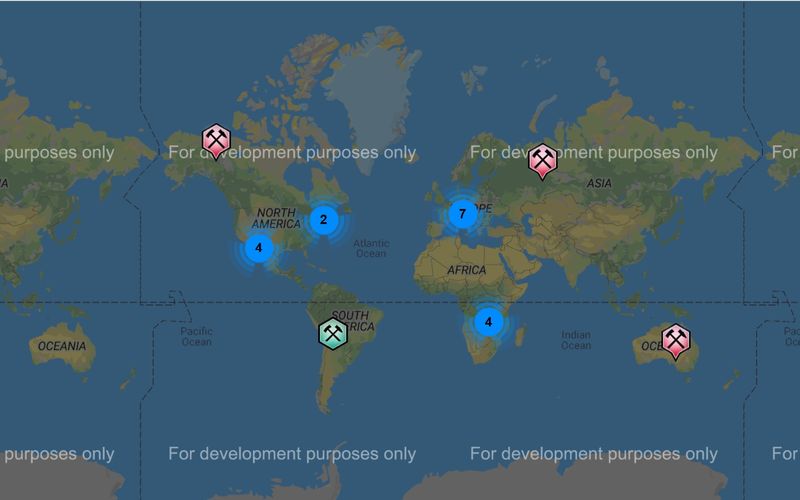
1. The geographic distribution of azurite: Attached are 2 screen shots of world azurite localities reported on Mindat ( the comparable one for malachite plots as "significant malachite occurrences, which is useless ...anyone knowing how to generate the apples to apples version please do so and post). First, azurite appears to concentrate at 35N and 30S latitudes...North American and Europe and Australia. Despite this concentration in (currently) temperate latitudes, our friends at the frozen extremes still enjoy a decent number of occurrences. Sorry Roger, but Belgium seems to fall on the north side of Main Street, so I'd posit that something else explains why you don't have much there. Geologically, azurite appears in all major copper fields: Porphyries, VMS, SED-hosted, with the notable exception of the enormous Zambian Belt deposits that are so rich in malachite but have very little azurite (reported). There are strong concentrations along the Cordilera of the western hemisphere and the Tethyan Belt, probably related to abundance of Porphyry Copper deposits in/near carbonate host rocks (Belgium is north of the Tethyan Belt as usually defined I think). Deposit age may be important...especially as related to length and depth of oxidation and erosion rate...as evidenced by a general lack of occurrences across northern Canada and eastern Russia where recent glaciation stripped off the oxidized surface...and both sub-Saharan Africa, the Amazon, and southern Asia where rainfall rates are high and humic acids flush carbonate (and almost everything else) out of the system.
2. Conditions of formation. As Vink points out (and others have echoed), azurite/malachite stabiity is very sensitive to PCO2 ...and the equilibrium between them is very close to that of normal atmosphere. Azurite falls on the losing side of this equilibrium most of the time, so unless the conditions become relatively acidic while the carbonate activity goes up, malachite is more likely to precipitate...or replace azurite after conditions return to "normal". The abudance of malachite pseudomorphs after azurite reflects this, but I think the repetitions of that paragenesis: where azurite covers malachite that pseudomorphed azurite (lather rinse repeat) provide important clues. I have pieces from Milpilas that show 2.5 repetitions (finishing as azurite) and I suspect more can be documented elsewhere. This indicates that conditions were fluctuating back and forth across that coincidental boundary (if you believe in coincidences) between the equilibrium PCO2 and normal atmospheric PCO2 ...and probably not moving too far away from it. Thus, relatively slight decreases in pH or increases in PCO2/carbonate activity would drive conditions temporarily into azurite stability, followed by a return to malachite precipitation. Given that both of these minerals occur in oxidizing sulfide-rich environments it's easy to see how those conditions could vary repeatedly over time as climatic and local conditions changed. Since the conditions for malachite stability are the status quo is it possible that conditions shifted incrementally into azurite stability and returned to malachite abruptly...perhaps providing another component in the "why not az ps mal" story?
3. Left Field Possibility: We are increasingly recognizing the importance of biological activity in the oxidation (and deposition) of sulfides and it is quite likely (virtually certain) that one or more bugs are involved in some way in our story here. Not sure how bugs would affect the redeposition of secondary copper minerals (there is no question they mediate the deposition of manganese oxides), so maybe the coincidence of atmospheric PCO2 and the az-mal PCO2 equilibrium somehow reflects their evolution under normal atmospheric PCO2 conditions?
4. Volume differences between azurite and malachite. Studies of the "electric blue" azurites from Milpillas show they are multi-stage pseudomorphs with a final layer of azurite over malachite ps azurite and that it is the ability of light to penetrate just the final skin of azurite and reflect off the underlying malachite that gives the intense blue color. These studies also show that there is a thin gap betwen the azurite and the malachite (some have suggested it is the gap that causes/enhances the electric blue effect) and that the underlying malachite is porous with a hackly and irregular surface. This echoes the earlier volume difference discussions, but might also suggest that at the onset of azurite deposition, the more acidic fluids that deposited the azurite first attacked/dissolved the malachite...perhaps more than we realize...removing any evidence of pseudomorphing by earliest azurite. Anybody know if similar textures have been noted elsewhere?
| Description: |
| Mindat map of world azurite localities |
|
| Viewed: |
29962 Time(s) |
|
| Description: |
| Mindat map of azurite localities focused on Europe and N Am |
|
| Viewed: |
28973 Time(s) |
|
| Description: |
| Mindat map of "selected" malachite localities...useless...does anyone know how to get the full map? |
|
| Viewed: |
29070 Time(s) |
|
_________________
Siempre Adelante! |
|
| Back to top |
|
 |
Peter Megaw
Site Admin

Joined: 13 Jan 2007
Posts: 975
Location: Tucson, Arizona



|
 Posted: Jun 07, 2019 17:38 Post subject: Re: Stability of Azurite vs Malachite Posted: Jun 07, 2019 17:38 Post subject: Re: Stability of Azurite vs Malachite |
|
|
One of my profs in university always told us to focus on the contacts because that's where things changed and where you'll find the evidence for the change...
Here's a remarkable piece from Milpillas...clearly all azurite originally but completely pseudomorphed to malachite on one side, with fresh azurite on the other. The altering fluids were clearly attacking the azurite from one side towards the other and the contact between them is an abrupt and sharp concave plane. This suggests that one side was bathed in fluids that provoked the pseudomorphing, while the other side was somehow isolated from that process.
A clear case of arrested development!!
| Mineral: | Azurite and malachite ps azurite |
| Locality: | | Milpillas Mine, Cuitaca, Municipio Santa Cruz, Sonora, Mexico |  |
|
| Dimensions: | 8 x 3 cm |
| Description: |
| Two sided specimen with azurite crystals on one side and malachite pseudomorphs after azurite (identical morphology) on the other. The contact between the two species is a sharp, but concave plane |
|
| Viewed: |
29004 Time(s) |

|
_________________
Siempre Adelante! |
|
| Back to top |
|
 |
Robert Seitz
Joined: 29 Dec 2015
Posts: 261
Location: Phoenix, AZ



|
 Posted: Jun 07, 2019 19:03 Post subject: Re: Stability of Azurite vs Malachite Posted: Jun 07, 2019 19:03 Post subject: Re: Stability of Azurite vs Malachite |
|
|
As much variability as this generates, it is interesting to consider results from addition of phosphate ions and silica ions; and the resulting stability fields of chrysocolla, libethenite, and pseudomalachite.
This is reported by Crane et al, Formation of chrysocolla and secondary copper phosphates in the highly weathered supergene zones of some Australian deposits, Records of the Australian Museum, 53, 2001, 49-56.
https://media.australianmuseum.net.au/media/Uploads/Journals/17903/1323_complete.pdf
(link normalized by FMF)
|
|
| Back to top |
|
 |
Peter Megaw
Site Admin

Joined: 13 Jan 2007
Posts: 975
Location: Tucson, Arizona



|
 Posted: Jun 07, 2019 19:13 Post subject: Re: Stability of Azurite vs Malachite Posted: Jun 07, 2019 19:13 Post subject: Re: Stability of Azurite vs Malachite |
|
|
Just what we need, two more degrees of freedom!!!
_________________
Siempre Adelante! |
|
| Back to top |
|
 |
Robert Seitz
Joined: 29 Dec 2015
Posts: 261
Location: Phoenix, AZ



|
 Posted: Jun 07, 2019 19:14 Post subject: Re: Stability of Azurite vs Malachite Posted: Jun 07, 2019 19:14 Post subject: Re: Stability of Azurite vs Malachite |
|
|
| It certainly makes for some interesting mineralization with oscillation of environment.
|
|
| Back to top |
|
 |
|





