| View previous topic :: View next topic |
| Author |
Message |
Josele

Joined: 10 Apr 2012
Posts: 410
Location: Tarifa, Spain



|
 Posted: Apr 25, 2021 10:19 Post subject: Calcite form Posted: Apr 25, 2021 10:19 Post subject: Calcite form |
|
|
I've been trying to determine Miller indices of a calcite specimen from Iraí, Brazil. I think it could be a combination of very steep rhombohedron, something like {0.16.1}, finished by the flat rhombohedron {012}. Big crystals seem be modified by an acute scalenohedron, maybe {541}. (Indices in accord to old calcite unit cell).
I will appreciate comments about this approach.
| Mineral: | Calcite |
| Locality: | | Iraí, Alto Uruguai region, Rio Grande do Sul, Brazil |  |
|
| Dimensions: | 9 x 6 x 3 cm |
| Description: |
| Plate of quartz covered by calcite tips |
|
| Viewed: |
8279 Time(s) |

|
| Mineral: | Quartz + Calcite |
| Locality: | | Iraí, Alto Uruguai region, Rio Grande do Sul, Brazil |  |
|
| Dimensions: | 9 x 6 x 3 cm |
| Description: |
| Backside is a mess of quartz crystals slightly covered by a late stage specie |
|
| Viewed: |
8275 Time(s) |

|
| Mineral: | Calcite |
| Locality: | | Iraí, Alto Uruguai region, Rio Grande do Sul, Brazil |  |
|
| Dimensions: | FoV: 4 cm |
| Description: |
|
| Viewed: |
8278 Time(s) |

|
| Mineral: | Calcite |
| Locality: | | Iraí, Alto Uruguai region, Rio Grande do Sul, Brazil |  |
|
| Dimensions: | FoV: 5 cm |
| Description: |
|
| Viewed: |
8272 Time(s) |

|
| Mineral: | Calcite |
| Locality: | | Iraí, Alto Uruguai region, Rio Grande do Sul, Brazil |  |
|
| Dimensions: | FoV: 5 cm |
| Description: |
|
| Viewed: |
8264 Time(s) |

|
| Mineral: | Quartz + Calcite |
| Locality: | | Iraí, Alto Uruguai region, Rio Grande do Sul, Brazil |  |
|
| Dimensions: | FoV in height: 5 cm |
| Description: |
| A quartz plate is the substrate of calcite tips |
|
| Viewed: |
8282 Time(s) |

|
|
|
| Back to top |
|
 |
Bob Carnein
Joined: 22 Aug 2013
Posts: 355
Location: Florissant, CO



|
 Posted: Apr 25, 2021 10:24 Post subject: Re: Calcite form Posted: Apr 25, 2021 10:24 Post subject: Re: Calcite form |
|
|
| Although I have no idea what the actual Miller indices are, I agree with your assignment of faces to those forms.
|
|
| Back to top |
|
 |
Pete Richards
Site Admin

Joined: 29 Dec 2008
Posts: 846
Location: Northeast Ohio



|
 Posted: Apr 25, 2021 12:00 Post subject: Re: Calcite form Posted: Apr 25, 2021 12:00 Post subject: Re: Calcite form |
|
|
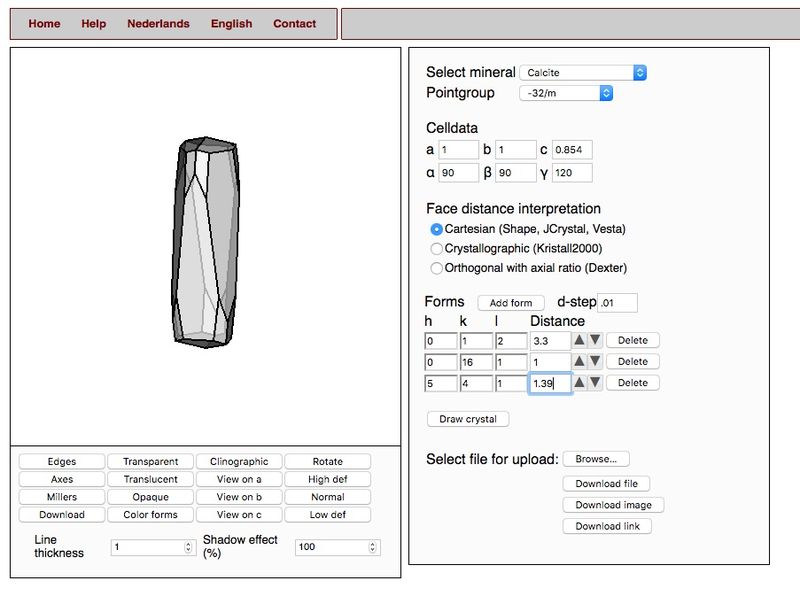
I think those forms are pretty reasonable guesses. {012} for sure. {0 16 1} is possible. {541} is about in the right place.
Attached is a smorf drawing I made of a crystal with these forms. Anyone who wants to can try out a crystal concept for himself at www.smorf.nl. It's easy and free!
| Description: |
|
| Viewed: |
8262 Time(s) |
|
_________________
Collecting and studying crystals with interesting habits, twinning, and epitaxy |
|
| Back to top |
|
 |
Josele

Joined: 10 Apr 2012
Posts: 410
Location: Tarifa, Spain



|
 Posted: Apr 25, 2021 15:53 Post subject: Re: Calcite form Posted: Apr 25, 2021 15:53 Post subject: Re: Calcite form |
|
|
Thanks for your comments.
A calcite dedicated page that for sure you know well was a great help for determining most common steep scalenohedron forms: http://nsminerals.atspace.com/calcite.html
Relating very steep rhombohedron, is hard distinguish {0.16.1} from {0.17.1} or even {0.20.1} without having very good crystals, a precise goniometer and the ability and training to do it.
Calcite is a generous mineral with hundreds if not thousands of crystal forms and enough common as to be affordable.
Anybody knows how many calcite forms are listed in Goldschmidt Atlas der Krystallformen?
There is any book exclusively dedicated to calcite crystals as it exists for fluorite?
|
|
| Back to top |
|
 |
Pete Richards
Site Admin

Joined: 29 Dec 2008
Posts: 846
Location: Northeast Ohio



|
 Posted: Apr 25, 2021 16:34 Post subject: Re: Calcite form Posted: Apr 25, 2021 16:34 Post subject: Re: Calcite form |
|
|
| Josele wrote: | (snip)...
Anybody knows how many calcite forms are listed in Goldschmidt Atlas der Krystallformen?
... |
It is necessary to distinguish between the term "form" in the strict crystallographic sense, and more common uses of the word to mean "shapes".
A form, sensu stricto, is a set of crystal faces that are all related to each other by the symmetry of the crystal. A cube is a form. A prism is a form, but there are two different ones for calcite. Each different rhombohedron is a form, as is each different scalenohedron. In an unpublished study of calcite in the 1940's, Charles Palache listed more than 700 forms for calcite, including more than 140 different rhombohedra and more than 400 different scalenohedra. He evaluated these in three groups - well established, probable, and questionable, based on several different factors including frequency of observation and quality of the faces involved.
Many crystals, including the one that is the subject of this thread, include more than one form, three in this case. Such a combination is best referred to as a "habit" or a "combination of forms". In German, the relevant term is "Tracht". With so many forms combined two, three, four or more together to produce a habit, the number of habits is conceptually infinite. Goldschmidt's Atlas depicts habits, and reports the forms that combine to produce them. The atlas has 2,544 drawings of calcite, though the number of illustrated habits is smaller because one habit may be shown in several drawings.
_________________
Collecting and studying crystals with interesting habits, twinning, and epitaxy |
|
| Back to top |
|
 |
Bob Morgan
Joined: 18 Jan 2018
Posts: 251
Location: Savannah, Georgia



|
 Posted: Apr 25, 2021 17:51 Post subject: Re: Calcite form Posted: Apr 25, 2021 17:51 Post subject: Re: Calcite form |
|
|
I've become more of a sceptic about so many forms. Tokody listed 459 for pyrite. Most are doubtful. Kostov wrote Calcite forms exceeded 620 (Crystal Habits of Minerals) I think I saw an article in German purporting to list them all. It was written in the 1930's.
More helpful is an article by Kenneth Brock "The Crystal Forms of Calcite" in the mineralogical Record, 24:Nov-Dec, 1993. The web site in the preceding entry is good too.
One test I use in looking at a face is to go into a dark room and flash a laser pointer on the surface and see what kind of reflection appears on a nearby white surface. If it is a relatively well defined point of reflection or a defined line or intersecting lines, there is a face. But in other instances there are large, spread out, weak blobs of light that I take to be indefinite surfaces made up of small curved surfaces but not faces. Yes, they have a relatively consistent overall angle and may be considered a face because of relatively overall flatness.
But often they resulted from some dissolution effects. An analogy would be like being sanded by a sanding block as the etching works consistently on a different angle of the crystal structure than growth, and also leaves pitting as well.
Calcite etches relatively easy. High Miller Index surfaces seem to be particularly prone to such irregularities.
Scalenahedral faces are often striated. Are these faces or stepped alternating faces? Their reflection will make a line perpendicular to the striations, hopefully with two nodes representing each of two alternating forms.
There is a lot of literature about the effect of impurities and impurity layers that help make less likely faces, but this is getting beyond my 'pay grade'.
Back to your original question: If you get a good reflection off the faces in question, a laser pointer can give accurate angle reads if set up right. It requires the reflection to be distinct over 60+ cm from the crystal to dampen out any slight irregularities of measurement.
If you decide to try this, I'll send some suggestions for set up.
What a topic!
|
|
| Back to top |
|
 |
Josele

Joined: 10 Apr 2012
Posts: 410
Location: Tarifa, Spain



|
 Posted: Apr 26, 2021 11:17 Post subject: Re: Calcite form Posted: Apr 26, 2021 11:17 Post subject: Re: Calcite form |
|
|
Thanks for these impressive calcite data. I suppose calcite is the specie with the largest number of documented combinations of basic forms. Is that true? More than fluorite?
This is another remarkable feature to point in calcite wonders list along with its optical properties, perfect cleavage, solubility inverse to temperature and so on.
In his priceless book "Krystallformen von Fluorit" Eddy Van Der Meersche tells that it took 25 years to gather and document the 85 combinations of basic forms presented in his book. If calcite is more prolific than fluorite, a comparable book for calcite seems unaffordable, it would take longer than a lifetime!
|
|
| Back to top |
|
 |
|





