| View previous topic :: View next topic |
| Author |
Message |
Elise

Joined: 22 Dec 2009
Posts: 243
Location: New York State



|
 Posted: Mar 11, 2013 09:33 Post subject: Re: Is this a twin? Posted: Mar 11, 2013 09:33 Post subject: Re: Is this a twin? |
|
|
Hi Pete, Duncan, John and all -
This morning I was reading an entry regarding emeralds in the Abstracts section of the most recent Gems & Gemology, Winter 2012, p. S6 : "Pressure-temperature-fluid constraints for the Emmaville-Torrington emerald deposit, New South Wales,Australia: Fluid inclusion and stable isotope studies." The G&G abstract stated: "The process of emerald precipitation, dissolution, and subsequent re-precipitation was evident in the disrupted, irregular zones shown by backscatter electron and CL images. These images also revealed Brazil-law twinning. "
....and my jaw dropped.
Thinkng that must be a typo, I went to the original paper by the author (see full paper reference below) - on page 292 it states " Brazilian twinning is also observed in the emerald crystal (Figure 7) showing intersectorial zoning, which could also be due to chemical starvation during quick growth of the crystal." The caption of Figure 7 on the same page is: "Photomicrographs of backscatter electron image versus
cathode luminescence image of the emerald crystal. Good correlation between light coloured (CL and BSE) and coloured beryl/emerald zones. Central zoning in centre
of image indicates emerald precipitation, dissolution and subsequent reprecipitation of the more parallel outer growth zones. The intersectorial zoning is also referred to
as Brazilian twinning."
The authors give a reference for "Brazilian twinning", but I did not chase it down (see below).
Is this paper suggesting that there is actual twinning in this beryl and that this "Brazilian twinning" is the same or similar to Brazil-law twinning in quartz? I haven't heard of the term "Brazilian twinning" (to me, this appears to be only color zoning due to composition changes) and if I'm correct that this a twist on the accepted term "Brazil-law", then I hope not to perpetuate a twisted term, but rather help halt its further usage.
I am fascinated by Brazil-law twinning and by quartz, but my understanding of how this very complex phenomenon is triggered and its formation doesn't jive with its possibility in beryl. ( some references on the bottom of my essay here http:(slash, slash)www(dot)nordskip(dot)com(slash)ametrine(dot)html) Is there any other mineral known to exhibit Brazil-law twinning?
Any insights welcome!
Elise
Pressure-temperature-fluid constraints for the Emmaville-Torrington emerald deposit, New South Wales,Australia: Fluid inclusion and stable isotope studies.L. Loughrey, D. Marshall, P. Jones, P. Millsteed,and A. Main, Central European Journal of Geo -sciences, Vol. 4, No. 2, pp. 287–299.
Author's reference for "Brazilian twinning" Ramseyer, K. and Müllis, J., Geologic application of cathodoluminescence of silicates. In M. Pagel, V. Barbin, P. Blanc, and D. Ohnenstetter,Eds., Cathodoluminescence in Geosciences, Springer-Verlag, Berlin, 2000, 177–191.
| Pete Richards wrote: | John White correctly pointed out that I missed some reported twin laws for beryl. Duncan (xenolithos) mentions three twin laws from Deer Howie and Zussman; these three are echoed in Dana's System 8th edition, listing the first two as rare and the last as doubtful.
John's mention of twinning on {hkil} apparently comes from the Handbook of Mineralogy. This is a singularly useless description (not John's fault!) because h, k, and l can represent any number (and i is always equal to h+k or -(h+k), depending on whether it is written with a superior bar or not).
(There is a growing tendency to omit the i index from Miller indices in the hexagonal system, because it is superfluous, and I do so below and did so in my first post but failed to mention it).
Of the three laws Duncan quotes, {110} is highly questionable since that plane is a symmetry element for beryl, and therefore twinning on that plane does nothing to change the structure or the morphology, which is what the concept of twinning is all about. This twin law only makes sense if some special structural modification (such as ordered impurities) reduces the symmetry; in any case if the law is known for such situations, it would not be visible in the external morphology.
So we are left (in my mind) with there being two possible twin laws, {311} and {401}, of which the first is deemed rare and the second doubtful by the authors of Dana 8. Until I see a convincing example, I'm dubious for sure!
In any case, both of those twin laws lead to a V-shaped morphology that looks nothing like the one that started this post, as the drawing below shows. |
_________________
Elise Skalwold |
|
| Back to top |
|
 |
Pete Richards
Site Admin

Joined: 29 Dec 2008
Posts: 843
Location: Northeast Ohio



|
 Posted: Mar 15, 2013 10:19 Post subject: Re: Is this a twin? Posted: Mar 15, 2013 10:19 Post subject: Re: Is this a twin? |
|
|
Elise,
I have no idea what "Brazilian twinning" is, and I am unable to access the journal that apparently contains the definition. I don't find anything on the web.
In any case, Brazil-law twinning in quartz is twinning by reflection across {11-20}. In beryl, {11-20} is a mirror plane in the beryl structure, so it cannot function as a twin plane. Reflection across {11-20} merely reproduces the structure - it does not produce anything new.
So if that's what Brazilian twinning in beryl is supposed to be, it's bogus.
In response to your other question, Brazil-law twinning could occur in other minerals with sufficiently low symmetry. However, such names usually just apply to given species, so even if another mineral had twinning by the same geometric relationship, it probably would not be called Brazil-law twinning.
_________________
Collecting and studying crystals with interesting habits, twinning, and epitaxy |
|
| Back to top |
|
 |
Roger Warin

Joined: 23 Jan 2013
Posts: 1232



|
 Posted: Apr 13, 2013 16:24 Post subject: Re: Is this a twin? Posted: Apr 13, 2013 16:24 Post subject: Re: Is this a twin? |
|
|
About Brazil Quartz twin.
I think there is a big difference in crystal structure between quartz and beryl.
Quartz is built on a three-dimensional framework (SiO2)n. For silica, variations in temperature and pressure easily lead to different crystalline phases, but they are still silica. Quartz is the low temperature phase of crystalline silica.
Twins often appear in the upper part of a domain of a phase stability. Disruption of the lattice may occur.
The Brazil twin is also called the Chiral Law. Therefore, in the structure, only the quartz chirality changes.
Starter is accustomed to seeing such twins formed from joining or penetration of two or more individuals. The Brazil twin is very common, but as internal twinning. In this case, individuals are not associated, but the inner zones of the crystal have different optical orientations. Composition planes of the Brazil twin separating the various fields become curved surfaces.
Roger.
|
|
| Back to top |
|
 |
Duncan Miller

Joined: 25 Apr 2009
Posts: 138
Location: South Africa



|
 Posted: May 15, 2019 07:31 Post subject: Re: Is this a twin? Posted: May 15, 2019 07:31 Post subject: Re: Is this a twin? |
|
|
Is this a morphological Brazil-law twin? It appears to have x-faces in both upper corners of the prism face.
| Mineral: | Quartz |
| Locality: | | Erongo Region, Namibia |  |
|
| Description: |
|
| Viewed: |
63713 Time(s) |

|
|
|
| Back to top |
|
 |
Pete Richards
Site Admin

Joined: 29 Dec 2008
Posts: 843
Location: Northeast Ohio



|
 Posted: May 15, 2019 08:40 Post subject: Re: Is this a twin? Posted: May 15, 2019 08:40 Post subject: Re: Is this a twin? |
|
|
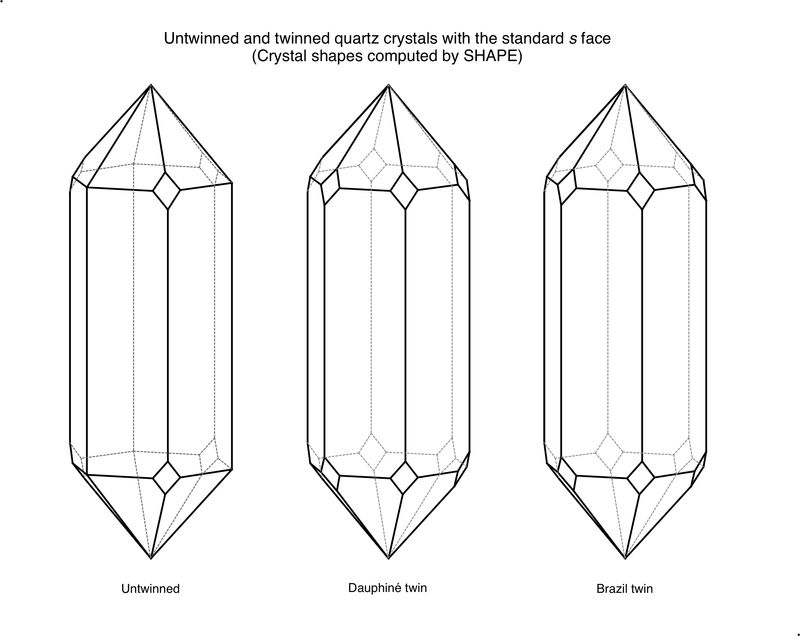
The morphology is consistent with Brazil=-law twinning, but is not compelling evidence for it. The crystal should be examined in light reflected from the various faces to look for evidence of differences within a face in its luster, which is indicative of a twin boundary. Dauphiné twin boundaries tend to be curvy, while Brazil twin boundaries tend to be marked by straight boundaries.
There are several problems with identifying this as a twin just based on the two little faces. One is the question of whether they are really x faces or whether they are s faces; this could only be resolved by goniometric measurement, since only one (x or s) is present. If they are s faces rather than x, they are symmetric across the prism edge, so their presence on two successive edges is indicative of twinning, but not diagnostic for which kind, Dauphiné or Brazil. To make matters worse, these faces could be present even in the absence of twinning - such faces are reported in the literature but are much less common than the true s and x faces.
So the frustrating reality is that this could be a Brazil-law twin, but the evidence of the two little faces is only suggestive, and confirmatory evidence is probably not available.
| Description: |
|
| Viewed: |
63598 Time(s) |
|
_________________
Collecting and studying crystals with interesting habits, twinning, and epitaxy |
|
| Back to top |
|
 |
Duncan Miller

Joined: 25 Apr 2009
Posts: 138
Location: South Africa



|
 Posted: May 15, 2019 09:33 Post subject: Re: Is this a twin? Posted: May 15, 2019 09:33 Post subject: Re: Is this a twin? |
|
|
Hello Pete - Thanks for your very prompt response. The crystal does have natural etching. Here is a photograph showing the opposite prism face. Curvy or straight, or should one use magnification?
| Mineral: | Quartz |
| Locality: | | Erongo Region, Namibia |  |
|
| Dimensions: | 60x25x20 mm |
| Description: |
|
| Viewed: |
19690 Time(s) |

|
|
|
| Back to top |
|
 |
Bob Morgan
Joined: 18 Jan 2018
Posts: 251
Location: Savannah, Georgia



|
 Posted: May 15, 2019 11:38 Post subject: Re: Is this a twin? Posted: May 15, 2019 11:38 Post subject: Re: Is this a twin? |
|
|
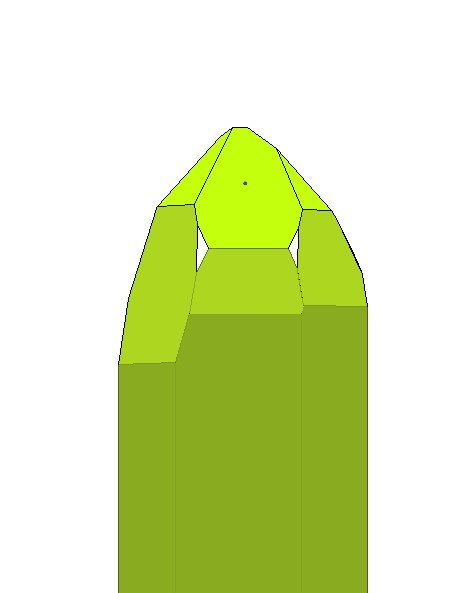
I doubt very much that this is a Brazil law twin. The two faces you propose as x faces are actually s faces. (See Drawing) They look like x faces because they are adjacent to large steep rhombohedral faces (the in between green faces).
With s faces on adjacent corners this is certainly a twin. As Pete said it could be either. Since clear evidence of Brazil Law twinning is lacking, it's more likely a Dauphene Law twin.
I'm sorry I can't get the drawing in this post. FOM doesn't allow the file.
Maybe Pete could tell me how to post a drawing.
| Description: |
|
| Viewed: |
19701 Time(s) |
|
|
|
| Back to top |
|
 |
Pete Richards
Site Admin

Joined: 29 Dec 2008
Posts: 843
Location: Northeast Ohio



|
 Posted: May 15, 2019 11:50 Post subject: Re: Is this a twin? Posted: May 15, 2019 11:50 Post subject: Re: Is this a twin? |
|
|
I thought maybe I saw that in the first image you posted, one the left side....
Those look like Dauphiné twin boundaries to me, sorry to say. Dauphiné-law twins are much more common, or much more commonly recognizable, than Brazil-law twins.
_________________
Collecting and studying crystals with interesting habits, twinning, and epitaxy |
|
| Back to top |
|
 |
Bob Morgan
Joined: 18 Jan 2018
Posts: 251
Location: Savannah, Georgia



|
 Posted: May 15, 2019 12:01 Post subject: Re: Is this a twin? Posted: May 15, 2019 12:01 Post subject: Re: Is this a twin? |
|
|
Duncan,
I just saw your second photo. That's definitely the sign of Dauphene Law twinning.
|
|
| Back to top |
|
 |
Duncan Miller

Joined: 25 Apr 2009
Posts: 138
Location: South Africa



|
 Posted: May 15, 2019 14:47 Post subject: Re: Is this a twin? Posted: May 15, 2019 14:47 Post subject: Re: Is this a twin? |
|
|
| Bob Morgan wrote: | I doubt very much that this is a Brazil law twin. The two faces you propose as x faces are actually s faces. (See Drawing) They look like x faces because they are adjacent to large steep rhombohedral faces (the in between green faces).
With s faces on adjacent corners this is certainly a twin. As Pete said it could be either. Since clear evidence of Brazil Law twinning is lacking, it's more likely a Dauphene Law twin. |
Bob, thank you for the drawing, which explains the little triangular faces well. I have several undoubted Dauphiné twin quartz crystals from the Erongo, with the characteristic patchy etching that one can follow across adjacent faces, but assumed these two little faces were x and not s. Wishful thinking!
|
|
| Back to top |
|
 |
Kevin Conroy

Joined: 03 Dec 2018
Posts: 78
Location: Saint Louis, Missouri


|
 Posted: May 15, 2019 14:56 Post subject: Re: Is this a twin? Posted: May 15, 2019 14:56 Post subject: Re: Is this a twin? |
|
|
Hi Pete,
It's been a LONG time since I sat in a mineralogy class, but for the life of me I can't see a difference between the Dauphiné and Brazil twin drawings. Could you please point out what I should be looking for?
Cheers,
Kevin
|
|
| Back to top |
|
 |
Bob Morgan
Joined: 18 Jan 2018
Posts: 251
Location: Savannah, Georgia



|
 Posted: May 15, 2019 16:45 Post subject: Re: Is this a twin? Posted: May 15, 2019 16:45 Post subject: Re: Is this a twin? |
|
|
Kevin,
You got the point. With only s faces they wouldn't look different. They could be either.
Duncan,
As regards to Dauphene twinning revealed by light etching - it's interesting that when a twin boundary goes around an edge from r to z the etch pattern switches. That's because on one side of the edge it's an r face and on the other a z face.
|
|
| Back to top |
|
 |
Kevin Conroy

Joined: 03 Dec 2018
Posts: 78
Location: Saint Louis, Missouri


|
 Posted: May 15, 2019 20:06 Post subject: Re: Is this a twin? Posted: May 15, 2019 20:06 Post subject: Re: Is this a twin? |
|
|
| Thanks Bob!
|
|
| Back to top |
|
 |
Duncan Miller

Joined: 25 Apr 2009
Posts: 138
Location: South Africa



|
 Posted: May 16, 2019 02:17 Post subject: Re: Is this a twin? Posted: May 16, 2019 02:17 Post subject: Re: Is this a twin? |
|
|
| Bob Morgan wrote: | | As regards to Dauphene twinning revealed by light etching - it's interesting that when a twin boundary goes around an edge from r to z the etch pattern switches. That's because on one side of the edge it's an r face and on the other a z face. |
Yes, that is very clear on several of my Erongo smoky quartz specimens. Amir Akhavan has a good photograph of an Erongo specimen showing this on his site The Quartz Page.
|
|
| Back to top |
|
 |
|




