| View previous topic :: View next topic |
| Author |
Message |
Jordi Fabre
Overall coordinator of the Forum

Joined: 07 Aug 2006
Posts: 5050
Location: Barcelona



|
 Posted: Oct 18, 2011 13:14 Post subject: Re: Fluorescence -again- Posted: Oct 18, 2011 13:14 Post subject: Re: Fluorescence -again- |
|
|
Maxilos,
I added the new thread you created to the previous thread about fluorescences in order to keep the topic more coherent.
|
|
| Back to top |
|
 |
Maxilos
Joined: 02 Nov 2010
Posts: 191
Location: Boskoop, The Netherlands



|
 Posted: Oct 18, 2011 15:09 Post subject: Re: Minerals and fluorescence Posted: Oct 18, 2011 15:09 Post subject: Re: Minerals and fluorescence |
|
|
So, Jesse you say that REE are causing this. Is it okay if I can call you from this moment a fluorite-specialist? But I got an other question very close related to the last one: lots of articles on the Internet say that uranium (U3+ or U6+) atoms, manganese (Mn2+ or Mn4+) atoms or a combination of these give a red or green fluorescence. Can you confirm any of this?
Mark
_________________
"Still looking for the philosopher's stone" => Dutch proverb |
|
| Back to top |
|
 |
Jesse Fisher

Joined: 18 Mar 2009
Posts: 639
Location: San Francisco



|
 Posted: Oct 20, 2011 09:47 Post subject: Re: Minerals and fluorescence Posted: Oct 20, 2011 09:47 Post subject: Re: Minerals and fluorescence |
|
|
| Unfortunately, I am not an expert on the causes of fluorescence. I have read that divalent Mn is thought to be responsible for a number of different fluorescent colors in minerals, including red/orange in calcite, and the green in willemite. Hexavalent uranium causes a yellow-green fluorescence in uranium glass and many U-bearing minerals from the oxide zones of uranium deposits. As to the details of who and why this happens, there are likely more knowledgeable folks out there than me.
|
|
| Back to top |
|
 |
Maxilos
Joined: 02 Nov 2010
Posts: 191
Location: Boskoop, The Netherlands



|
 Posted: Oct 21, 2011 13:16 Post subject: Re: Minerals and fluorescence Posted: Oct 21, 2011 13:16 Post subject: Re: Minerals and fluorescence |
|
|
Well, I was thinking at my work: If pure fluorite is colourless, it has no "contaminations" of other elements! Mayby because of the not correct charges of the atoms that have contaminated the fluorite. With this I mean in -lets say yttrocerite- that CeF2 or YF2 exist in minime quantities but the charges arn't the same:
Cerium has Ce3+ or Ce4+ ==> they both appear in the fluorite which makes Ce3+ F1-2 which means that there is space for one other electron. Same with all the other impurities.
Hope you like my theory,
Mark
_________________
"Still looking for the philosopher's stone" => Dutch proverb |
|
| Back to top |
|
 |
bjland4
Joined: 15 Nov 2011
Posts: 1
Location: Beijing


|
 Posted: Nov 15, 2011 01:25 Post subject: Re: Minerals and fluorescence Posted: Nov 15, 2011 01:25 Post subject: Re: Minerals and fluorescence |
|
|
Yes ! I agree this !
Minerals forming from a solution - A solution, running through rocks, carries a dissolved load. If something changes (temperature, concentration of solutes in the solution because the solution encounters other water or a different rock type, etc.) then the solution may precipitate a mineral. Again, the mineral forms only if by forming the mineral, the energy of the system decreases. Evaporation is a simple case of minerals forming from solution - a particular mineral will start to precipitate from an evaporating solution when energetically favorable to do so.
As to fluorescence, atoms in minerals have electrons. These electrons can inhabit different energy states. Like mineral formation, nature like to be in the lowest energy state. But in this case, a photon (light) strikes the electron, causing it to jump to a higher energy state. When the electron falls back to its original state, it emits another photon with energy equal to the distance of the fall between the excited state and its base or ground state. If the photons of light hitting a mineral are of a too energetic for us to see, but the energy of the photons given off when the electrons fall back to their ground state is such that we can see them, then we call this fluorescence.
|
|
| Back to top |
|
 |
Maxilos
Joined: 02 Nov 2010
Posts: 191
Location: Boskoop, The Netherlands



|
 Posted: Nov 15, 2011 13:39 Post subject: Re: Minerals and fluorescence Posted: Nov 15, 2011 13:39 Post subject: Re: Minerals and fluorescence |
|
|
Wow, this is weird. That was just something I came up with and it could actually be correct! Thanks for supporting my theory!
Other theories are welcome too!
Mark
_________________
"Still looking for the philosopher's stone" => Dutch proverb |
|
| Back to top |
|
 |
Roger Warin
Joined: 23 Jan 2013
Posts: 1233



|
 Posted: May 20, 2013 06:26 Post subject: Luminescence - Fluorescence Posted: May 20, 2013 06:26 Post subject: Luminescence - Fluorescence |
|
|
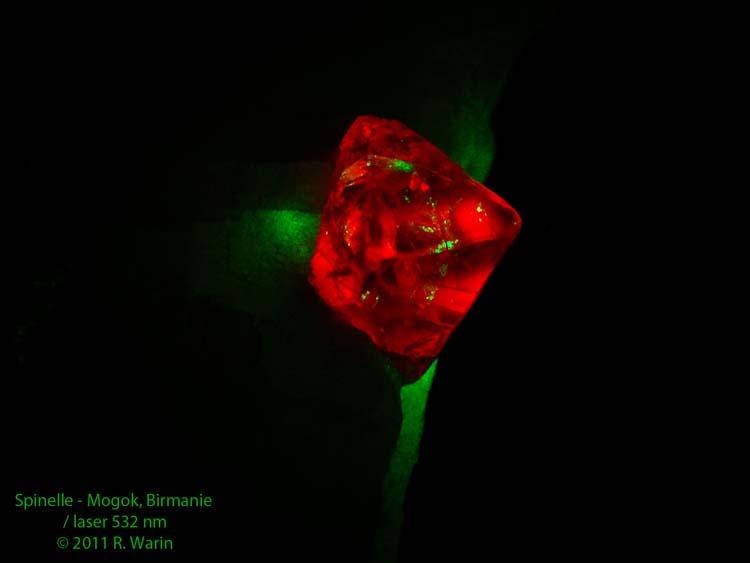
Hello,
Fluorescence can be initiated by photons of different energy (frequency). There are not only long and short UV rays that induce luminescence. Here spinel excited by a green laser (532 nm).
Spinel from Mogok, Myanmar.
Roger.
| Description: |
Spinel
Mogok, Myanmar
8 mm
Fluorescence with a green laser |
|
| Viewed: |
32773 Time(s) |
|
|
|
| Back to top |
|
 |
Riccardo Modanesi
Joined: 07 Nov 2011
Posts: 631
Location: Milano


|
 Posted: May 20, 2013 07:53 Post subject: Re: Luminescence - fluorescence Posted: May 20, 2013 07:53 Post subject: Re: Luminescence - fluorescence |
|
|
Hi Roger!
Interesting! How can I get this kind of light? Is it commonly available or is it just a laboratory device? If the former possibility is right, it would be interesting as an analysis instrument for faceted or cut stones as well!
Greetings from Italy by Riccardo.
_________________
Hi! I'm a collector of minerals since 1973 and a gemmologist. On Summer I always visit mines and quarries all over Europe looking for minerals! Ok, there is time to tell you much much more! Greetings from Italy by Riccardo. |
|
| Back to top |
|
 |
Jordi Fabre
Overall coordinator of the Forum

Joined: 07 Aug 2006
Posts: 5050
Location: Barcelona



|
 Posted: May 20, 2013 10:11 Post subject: Re: Luminescence - Fluorescence Posted: May 20, 2013 10:11 Post subject: Re: Luminescence - Fluorescence |
|
|
| Roger Warin wrote: | Hello,
Fluorescence can be initiated by photons of different energy (frequency). There are not only long and short UV rays that induce luminescence. Here spinel excited by a green laser (532 nm).
Spinel from Mogok, Myanmar.
Roger. |
Posts merged to this other thread: Minerals and fluorescence for better visibility and coherence.
|
|
| Back to top |
|
 |
Roger Warin

Joined: 23 Jan 2013
Posts: 1233



|
 Posted: May 20, 2013 10:54 Post subject: Re: Minerals and fluorescence Posted: May 20, 2013 10:54 Post subject: Re: Minerals and fluorescence |
|
|
Hello Ricardo,
This is not problem. The laser comes from China (10 or € 15).
The only problem is to fix the device switched pointing spinel.
Proficiat.
Roger.
| Description: |
|
| Viewed: |
32613 Time(s) |

|
| Description: |
|
| Viewed: |
32630 Time(s) |

|
|
|
| Back to top |
|
 |
chris
Site Admin

Joined: 12 Jul 2007
Posts: 538
Location: Grenoble



|
 Posted: May 20, 2013 11:28 Post subject: Re: Minerals and fluorescence Posted: May 20, 2013 11:28 Post subject: Re: Minerals and fluorescence |
|
|
Hi Roger,
Maybe I'm a bit paranoid on that, but is it a good idea to illuminate mineral specimens with laser beam ? of course the light produced by such device is far less energetic than the energy of lab or military lasers, but I'm wondering if exposing colored minerals to laser beam could damage them knowing the light remains much stronger than daylight light ?
As discussed in this thread: How to minimize Arsenic on Realgar, I don't think it would be wise to use such green light laser on realgar...
Any idea on that ?
Chris
|
|
| Back to top |
|
 |
Roger Warin

Joined: 23 Jan 2013
Posts: 1233



|
 Posted: May 20, 2013 15:51 Post subject: Re: Minerals and fluorescence Posted: May 20, 2013 15:51 Post subject: Re: Minerals and fluorescence |
|
|
Hello,
This is a very interesting question.
From a fundamental point of view, the physical chemist is not interested in the stability of the molecule when it is analyzed. Everything depends on the technique used.
However, the luminescence is a gentle technique. It does not destroy the sample in any way. The luminescence emission gives a cold light, unlike incandescent one, which gives an emission spectrum, which corresponds to a warm light.
Photons used with a green laser cause a small excitation of some electrons in the atom or molecule, which fall to the original level by emitting energy. In the case of luminescence (or fluorescence), this form of light energy appears but with a change in wavelength. The invisible UV excitation light becomes visible (blue, red, yellow ...).
The case of realgar (As4S4) is very different. The light energy from the sun is sufficient to initiate a chemical decomposition (redox). We finally obtain orpiment (As2S3), but accompanied by As2O3 with the presence of air. But the situation is very complex, because it also gets in the early stages, some polymorphs of realgar as pararealgar (different molecule from orpiment). This aspect of inorganic chemistry is very complex.
In fact, realgar is a rather unstable crystal structure and a small supply of energy like light, can destroy it.
It is very far from the situation of a simple, highly resistant molecule as spinel (this mineral is refractory to high pressures and temperatures) slightly tickled by relatively small energetic photons (X-ray fluorescence is also possible).
The fluorescence excitation is a phenomenon which lasts from 10-9 to 10-6 seconds.
Roger.
|
|
| Back to top |
|
 |
Mark Ost

Joined: 18 Mar 2013
Posts: 516
Location: Virginia Beach



|
 Posted: May 20, 2013 19:04 Post subject: Re: Minerals and fluorescence Posted: May 20, 2013 19:04 Post subject: Re: Minerals and fluorescence |
|
|
Hi Roger. I have wanted to do this with my Mogok spinel and will get around to it this week end. Also wanted to try with a couple of ruby conundrums I have. Odd though. If you get the electron too energized it may jump a couple of levels and dissipate the energy of it falling in the form of heat or lattice vibration vice photon emission. I love the picture.
Ricardo, any astronomy supplier has these. I am a bit leery of some of the really powerful ones that some folks have made. Far too powerful for responsible use.
|
|
| Back to top |
|
 |
Roger Warin

Joined: 23 Jan 2013
Posts: 1233



|
 Posted: May 20, 2013 22:51 Post subject: Re: Minerals and fluorescence Posted: May 20, 2013 22:51 Post subject: Re: Minerals and fluorescence |
|
|
Hello Mark,
I cannot explain here the details of the fluorescence. The excited molecule returns to its stable level by several processes (heat dissipation, vibration and rotation ...). But it happens that the radiative energy is dissipated. First, small electron energy dissipation occur before the last jump (fall) to give the fluorescent photon. It is due to what is called as the Stokes shift.
In general, there is no fluorescence because the dissipation is so trivial by the general process and because there are no activators.
Some atoms promote fluorescence, such as REE or manganese. The site manager must possess many vacant orbitals poorly differentiated. Cerium is a frequent activator (fluorophore center). And as it is abundant on Earth and its steric hindrance is similar to that of calcium, it often replaces it in the crystal lattice.
If there are activators of fluorescence, there are also poisons of this phenomenon. They are inhibitors of fluorescence. They are common and they affect the immediate environment of the fluorophore center. Fluorescence is a random phenomenon in practice in geology.
The fluorescence is a technique widely used in biophysics in the study of proteins.
Roger.
|
|
| Back to top |
|
 |
Mark Ost

Joined: 18 Mar 2013
Posts: 516
Location: Virginia Beach



|
 Posted: May 21, 2013 03:15 Post subject: Re: Minerals and fluorescence Posted: May 21, 2013 03:15 Post subject: Re: Minerals and fluorescence |
|
|
| You are right on about random process. I don't recall any time spent on fluorescence in school. I suspect, like color, it is so highly variable in minerals it was shelved in favor of more certain techniques for mineral identification. It is a fascinating quality though and I have an entire case dedicated to it.
|
|
| Back to top |
|
 |
|




