| View previous topic :: View next topic |
| Author |
Message |
Paul Biehler
Joined: 12 Jan 2013
Posts: 30
Location: Northern California


|
 Posted: Apr 11, 2013 18:49 Post subject: Question about Azurite and Malachite Posted: Apr 11, 2013 18:49 Post subject: Question about Azurite and Malachite |
|
|
| I have been wondering why we don't see great Azurite and Malachite from the large copper deposits in Chile? What are the geological reasons? If there are Azurite and Malachite from Chile where would I be able to see them? Thank You Paul
|
|
| Back to top |
|
 |
alfredo
Site Admin

Joined: 30 Jan 2008
Posts: 1014



|
 Posted: Apr 11, 2013 19:58 Post subject: Re: Question about Azurite and Malachite Posted: Apr 11, 2013 19:58 Post subject: Re: Question about Azurite and Malachite |
|
|
| Part of the reason is that copper carbonates like azurite and malachite rarely form in abundance unless there is an abundant source of carbonate - ie. calcite and/or dolomite - in the primary sulphide ores. If calcite and dolomite are scarce, one ends up with copper sulphates, like the abundant antlerite in Chuquicamata, rather than azurte and malachite.
|
|
| Back to top |
|
 |
Jean Sendero

Joined: 20 Dec 2009
Posts: 270
Location: Hudson Heights, Quebec



|
 Posted: Apr 12, 2013 07:41 Post subject: Re: Question about Azurite and Malachite Posted: Apr 12, 2013 07:41 Post subject: Re: Question about Azurite and Malachite |
|
|
As Alfredo said, the development of malachite and azurite is dependent on the host rock into which the porphyry will intrude and form the deposits. In Chile, most of the porphyry Cu are formed in the Paleocene or Oligocene or Miocene volcanic arcs. The intrusions are in placed generally in coeval volcanic rocks or older volcaniclastic or intrusive rocks. Hence these two hosts are carbonate poor hence the rare occurrence of malachite and azurite.
In what the miners call the "oxide zone" located just above the secondary enriched sulphide zone, the most common secondary "green minerals" are brochantite, atacamite and chrysocolla. Additionally, due to the rare open space filling in porphyry Cu, most of these minerals will be deposited along fracture planes and very very rarely in cavities to form nice crystals. MinDat has a very nice photograph of brochantite on chrysocolla from Cerro Colorado located east of Iquique.
Few specimens make their way to the market from these deposits for a couple of reasons. First is the "mineral collecting" culture is poorly developed to un-existant in Chile. Hence, it is rarely recognized by those who work in these giant open pits that these can be valuable. Second, the mining companies, for multiple safety reasons, make it very difficult to retrieve specimens during the mining process.
Later this weekend, I will post a few photos illustrating some nice brochantite in cavity within massive enargite veins from Cerro Colorado, some cuprite and native copper from Cerro Colorado and some typical atacamite from Minera Spence.
Jean
|
|
| Back to top |
|
 |
Paul Biehler
Joined: 12 Jan 2013
Posts: 30
Location: Northern California


|
 Posted: Apr 12, 2013 10:32 Post subject: Re: Question about Azurite and Malachite Posted: Apr 12, 2013 10:32 Post subject: Re: Question about Azurite and Malachite |
|
|
Thank you so much Alfredo and Jean. I thought if might be partly do to the Andes being volcanic. I appreciate such fine explanations. Maybe you can help with another question. Why do so many fine minerals come from desert regions? Is it the geology or just easy to prospect there, or both.
Thank You again Paul
|
|
| Back to top |
|
 |
Riccardo Modanesi
Joined: 07 Nov 2011
Posts: 631
Location: Milano


|
 Posted: Apr 15, 2013 07:32 Post subject: Re: Question about Azurite and Malachite Posted: Apr 15, 2013 07:32 Post subject: Re: Question about Azurite and Malachite |
|
|
Hi to everybody!
maybe the reason why malachite and azurite are so scarce is to be researched not in a theoretical lack of oxygene, but in a more likely lack of carbon. Atacamite, for example, IS a well-oxygenated copper mineral and IS abundant in the Atacama desert in Chile (its name comes from there!), but it is a copper phosphate! Take it just as a personal theory, but, as we say in my country, "two plus two doesn't give five"!
Greetings from Italy by Riccardo.
_________________
Hi! I'm a collector of minerals since 1973 and a gemmologist. On Summer I always visit mines and quarries all over Europe looking for minerals! Ok, there is time to tell you much much more! Greetings from Italy by Riccardo. |
|
| Back to top |
|
 |
Joaquin Montoro
Joined: 25 Nov 2010
Posts: 225
Location: Murcia (Spain)



|
 Posted: Apr 15, 2013 08:24 Post subject: Re: Question about Azurite and Malachite Posted: Apr 15, 2013 08:24 Post subject: Re: Question about Azurite and Malachite |
|
|
Sorry Riccardo, but atacamite is not a copper phosphate...it is a basic chloride.
Cu2(OH)3Cl
_________________
Damnati ad Metalla |
|
| Back to top |
|
 |
Roger Warin

Joined: 23 Jan 2013
Posts: 1246



|
 Posted: Apr 15, 2013 10:27 Post subject: Re: Question about Azurite and Malachite Posted: Apr 15, 2013 10:27 Post subject: Re: Question about Azurite and Malachite |
|
|
About the origin of secondary copper minerals.
Ore generally consist of copper sulfides. In the superficial areas of the deposit, a hydrothermal weathering occurs. These chemical reactions set in motion copper cations. These can then be combined with other anions forming another insoluble compound. Another mineral species crystallizes.
Depending on conditions, cations combine with carbonates to form especially malachite and / or azurite according to environmental conditions (pH, etc.). In arid areas, the lack of water disrupts this process. Others anions as chlorides can then play a role
At Tsumeb, probably one of the best places for azurite, these crystals can be found even under 1000 m of rock as it exists on this site a big fault for the passage of water at great depths.
Roger.
|
|
| Back to top |
|
 |
Jean Sendero

Joined: 20 Dec 2009
Posts: 270
Location: Hudson Heights, Quebec



|
 Posted: Apr 15, 2013 22:57 Post subject: Re: Question about Azurite and Malachite Posted: Apr 15, 2013 22:57 Post subject: Re: Question about Azurite and Malachite |
|
|
Well, some interesting comments above that may need some clarity.
This topic is very very well documented and well understood by the geoscientists. I would refer to most of you, to a very good little article, and simple enough for everyone to understand its content. the reference is below:
Chavez, W.X., 2000, Supergene Oxidation of Copper Deposits: Zoning and distribution of Copper Oxides Minerals. SEG newsletter, No 41, p. 1-13. (you may be able to find it on the internet)
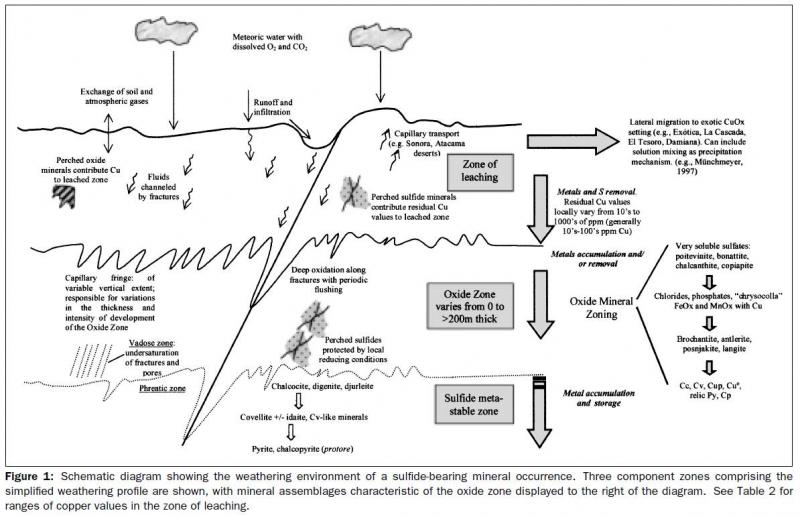
Below I have extracted one figure that explains a few things. This article refers to porphyry copper style mineralization but the physical and chemical reactions and conditions are similar to any other secondary mineral deposit. (i.e Mapimi, Sta Eulalia, Tsumeb, etc...)The rule is simple, pyrite is oxidized, creates some acid, mixed with water and leach the metals, travel downwards until the right conditions are met to precipitate the minerals. It is more complex but this is my one line explanation.
Below I have inserted photos progressing down into the weathering profile. 1) leach cap, (pyrite gone), 2) a photo of precipitated secondary minerals in the oxidized zone (in this case atacamite, veins and along fractures), 3) More advance oxidation with Cuprite, and 4) and one with the secondary sulphide mineralization (Chalcocite coating pyrite) at the water table.
Cheers
jean
| Description: |
Weathering Environment
see Chavez 2000 |
|
| Viewed: |
17928 Time(s) |
|
| Description: |
Leach cap
Spence Mine, 45 km south of Chuquicamata, El Loa Province, Chile
12 cm across
porphyry intrusion with casts of original sulphides (chalcopyrite, pyrite) Left behind is hematite, jarosite, and goethite. |
|
| Viewed: |
17911 Time(s) |

|
| Description: |
Leach cap close-up
Spence Mine, 45 km south of Chuquicamata, El Loa Province, Chile
7 cm across
same as previous but clearly showing the relic casts of chalcopyrite-pyrite. |
|
| Viewed: |
17957 Time(s) |

|
| Description: |
Atacamite
Spence Mine, 45 km south of Chuquicamata, El Loa Province, Chile
15 cm across
Vein of secondary atacamite in the oxidized host porphyry. Rare sulphide grains |
|
| Viewed: |
17920 Time(s) |

|
| Description: |
Atacamite
Spence Mine, 45 km south of Chuquicamata, El Loa Province, Chile
11 cm across
Atacamite coating a fracture. Crystals are ill-defined but for a few places. |
|
| Viewed: |
18032 Time(s) |

|
| Description: |
Brochantite on enargite
Cerro Colorado Mine, Province of Iquique, Chile
8 cm across
Late secondary brochantite associated with massive enargite veins |
|
| Viewed: |
17985 Time(s) |

|
| Description: |
Brochantite on enargite
Cerro Colorado Mine, Province of Iquique, Chile
5 cm across
close-up. Not huge crystals like at Milpillas, but still nice. |
|
| Viewed: |
17918 Time(s) |

|
| Description: |
Cuprite with native copper
Cerro Colorado Mine, Province of Iquique, Chile
8 cm tall |
|
| Viewed: |
17933 Time(s) |

|
| Description: |
Chalcocite coating pyrite
Spence Mine, 45 km south of Chuquicamata, El Loa Province, Chile
13 cm
Porphyry cut by quartz-pyrite veins with a thin coating of black chalcocite or djurliete on the pyrite. Both in the veins and as dissemination in the porphyry |
|
| Viewed: |
17926 Time(s) |

|
|
|
| Back to top |
|
 |
Riccardo Modanesi
Joined: 07 Nov 2011
Posts: 631
Location: Milano


|
 Posted: Apr 16, 2013 07:37 Post subject: Re: Question about Azurite and Malachite Posted: Apr 16, 2013 07:37 Post subject: Re: Question about Azurite and Malachite |
|
|
Hi Joaquin!
Of course you are right: this time I mistook it by thinking of a copper phosphate, therefore I apologize for my mistake; however turquoise and ceruleite (resp. copper phosphate and copper arseniate) ARE common in the Atacama Desert as well! And this time I'm sure: they ARE oxygenated copper minerals!
Greetings from Italy by Riccardo.
| Joaquin Montoro wrote: | Sorry Riccardo, but atacamite is not a copper phosphate...it is a basic chloride.
Cu2(OH)3Cl |
_________________
Hi! I'm a collector of minerals since 1973 and a gemmologist. On Summer I always visit mines and quarries all over Europe looking for minerals! Ok, there is time to tell you much much more! Greetings from Italy by Riccardo. |
|
| Back to top |
|
 |
Paul Biehler
Joined: 12 Jan 2013
Posts: 30
Location: Northern California


|
 Posted: Apr 16, 2013 19:01 Post subject: Re: Question about Azurite and Malachite Posted: Apr 16, 2013 19:01 Post subject: Re: Question about Azurite and Malachite |
|
|
Thank you again for a great explanation. My understanding is much more complete.
Paul
|
|
| Back to top |
|
 |
|




