| View previous topic :: View next topic |
| Author |
Message |
Oliver B
Joined: 30 Jul 2018
Posts: 27
Location: Columbus, Ohio


|
 Posted: Aug 04, 2018 11:58 Post subject: Re: Collection Macros Posted: Aug 04, 2018 11:58 Post subject: Re: Collection Macros |
|
|
| Hardness test of 4 after my tests, That's why they are so interesting to me; I wonder why their structure changed, I concluded that it had to be Fluorite. Thanks bob!
|
|
| Back to top |
|
 |
Oliver B
Joined: 30 Jul 2018
Posts: 27
Location: Columbus, Ohio


|
 Posted: Aug 04, 2018 12:04 Post subject: Re: Collection Macros Posted: Aug 04, 2018 12:04 Post subject: Re: Collection Macros |
|
|
Bob Harman,
I will post a few pictures of the geode I collected. I also thought it very strange to be in the river. I almost thought it was a discarded geode someone else collected or lost from another locale, however it wasn't cracked open yet.
| Mineral: | Quartz |
| Locality: | | Alum Creek, Columbus, Franklin County, Ohio, USA |  |
|
| Dimensions: | 10cm x 10cm |
| Description: |
| some more complete shots of the specimen |
|
| Viewed: |
32105 Time(s) |

|
| Mineral: | Quartz |
| Locality: | | Alum Creek, Columbus, Franklin County, Ohio, USA |  |
|
| Dimensions: | 10cm x 10cm |
| Description: |
|
| Viewed: |
32104 Time(s) |

|
| Mineral: | Quartz |
| Locality: | | Alum Creek, Columbus, Franklin County, Ohio, USA |  |
|
| Dimensions: | 10cm x 10cm |
| Description: |
| area shot containing the scepter of quartz |
|
| Viewed: |
32124 Time(s) |

|
|
|
| Back to top |
|
 |
Bob Carnein
Joined: 22 Aug 2013
Posts: 355
Location: Florissant, CO



|
 Posted: Aug 04, 2018 12:15 Post subject: Re: Collection Macros Posted: Aug 04, 2018 12:15 Post subject: Re: Collection Macros |
|
|
| Hi, again--can you tell me how you tested the hardness on the Alum Creek fluorites?
|
|
| Back to top |
|
 |
Bob Harman
Joined: 06 Nov 2015
Posts: 765



|
 Posted: Aug 04, 2018 12:27 Post subject: Re: Collection Macros Posted: Aug 04, 2018 12:27 Post subject: Re: Collection Macros |
|
|
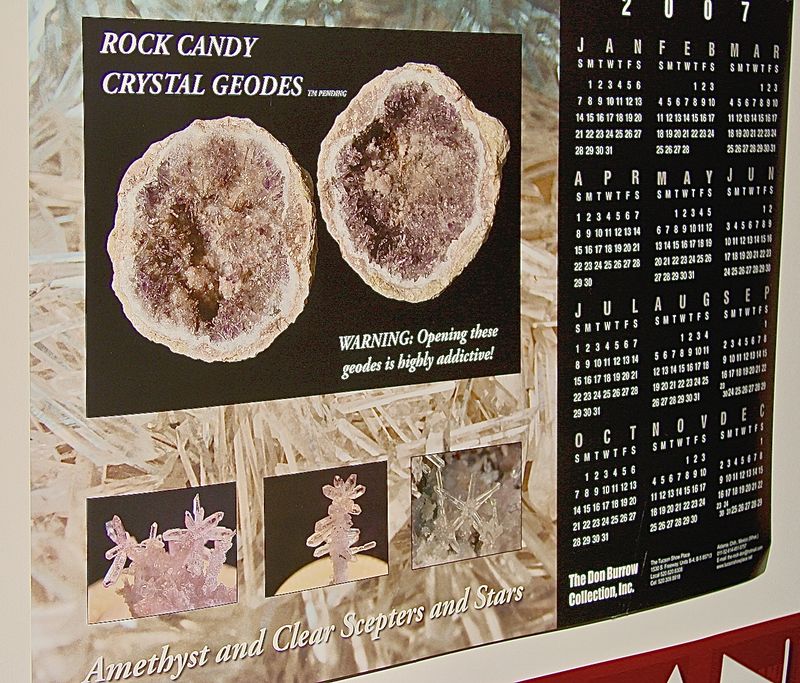
OLIVER, Your last 3 photos almost certainly show a Mexican geode of the type I have pictured. These were marketed as Rock Candy Geodes at the 2007 Tucson show. They are quite similar to yours with starburst like groups of small sceptered quartz crystals. I have the example pictured in the poster.
Unless there are other very similar examples definitely associated with the Olentangy River Ohio locality, finding your example suggests it was discarded by someone in the past. Look around in that area again and maybe you will be lucky with additional finds!
BOB
| Description: |
|
| Viewed: |
32077 Time(s) |
|
|
|
| Back to top |
|
 |
Oliver B
Joined: 30 Jul 2018
Posts: 27
Location: Columbus, Ohio


|
 Posted: Aug 04, 2018 13:07 Post subject: Re: Collection Macros Posted: Aug 04, 2018 13:07 Post subject: Re: Collection Macros |
|
|
| Bob Carnein, I first tested the very tip of the crystal with my fingernail and it left visible scratches on my nail, following up I used a copper plated penny and found that it also scratched the copper. Finally I took a carbon steel knife and found that it scratched very easily on the fluorite. Combined with the fluorescence and the fact that the other crystals in the same rock dump were easily distinguishable as calcite with dog tooth spar formation, I drew the conclusion that it was Fluorite. I hope my hardness test was satisfactory.
|
|
| Back to top |
|
 |
Chris Rayburn
Joined: 07 Oct 2013
Posts: 66
Location: Arvada, Colorado


|
 Posted: Aug 05, 2018 07:12 Post subject: Re: Collection Macros Posted: Aug 05, 2018 07:12 Post subject: Re: Collection Macros |
|
|
| A very simple and definitive test--place a couple of drops of vinegar on it. If it fizzes, it's calcite; if not, it's fluorite or something else.
|
|
| Back to top |
|
 |
Oliver B
Joined: 30 Jul 2018
Posts: 27
Location: Columbus, Ohio


|
 Posted: Aug 05, 2018 09:24 Post subject: Re: Collection Macros Posted: Aug 05, 2018 09:24 Post subject: Re: Collection Macros |
|
|
I will perform an acid test later today using vinegar. I have some pool based muriatic acid here that is around 30% concentration, on other samples to try and clean I mixed a 10:1 ratio of water and muriatic acid to remove calcite buildup and rust staining but what ended up happening was the crystals of fluorite in solution also began to loose their definitive edges and took on a glossy smooth appearance. I was under the impression that dilute HCL would not affect Fluorite crystals. Did i not dilute enough or would vinegar be the best solution? I have many nodules of pyrite from the area as well and wonder would the acid remove rust deposits?
| Mineral: | Fluorite with Calcite |
| Locality: | | Ohio, USA |  |
|
| Dimensions: | 10cm x 10cm |
| Description: |
| Glossy luster on the areas treated with HCL (Muriatic) |
|
| Viewed: |
15476 Time(s) |

|
| Mineral: | Fluorite |
| Locality: | | Ohio, USA |  |
|
| Dimensions: | 5cm X 5cm |
| Description: |
|
| Viewed: |
15477 Time(s) |

|
| Mineral: | Pyrite |
| Dimensions: | 5cm x 5cm |
| Description: |
|
| Viewed: |
15486 Time(s) |

|
|
|
| Back to top |
|
 |
Tom Tucker
Joined: 03 Dec 2009
Posts: 60
Location: Virginia


|
 Posted: Aug 05, 2018 13:56 Post subject: Re: Collection Macros Posted: Aug 05, 2018 13:56 Post subject: Re: Collection Macros |
|
|
| I think the crystals in "the glossy area" are calcite that has been actively dissolved by the HCl. I don't see any fluorite. Tom
|
|
| Back to top |
|
 |
Chris Rayburn
Joined: 07 Oct 2013
Posts: 66
Location: Arvada, Colorado


|
 Posted: Aug 05, 2018 15:30 Post subject: Re: Collection Macros Posted: Aug 05, 2018 15:30 Post subject: Re: Collection Macros |
|
|
| Agree, looks like acid etched calcite. Dilute HCl and vinegar should have a similar effect on calcite, although dilute HCl will generally be more aggressive. HCl should have no effect on fluorite. I've had good luck cleaning pyrite nodules with HCl, but it depends largely on what type of material is coating the pyrite. It should not harm the pyrite in any event.
|
|
| Back to top |
|
 |
Bob Harman
Joined: 06 Nov 2015
Posts: 765



|
 Posted: Aug 05, 2018 15:58 Post subject: Re: Collection Macros Posted: Aug 05, 2018 15:58 Post subject: Re: Collection Macros |
|
|
| If these are all the same examples that I originally commented on, then I was correct as they look like acid etched calcite. I therefore agree with Chris and Tom. BOB
|
|
| Back to top |
|
 |
Oliver B
Joined: 30 Jul 2018
Posts: 27
Location: Columbus, Ohio


|
 Posted: Aug 05, 2018 16:25 Post subject: Re: Collection Macros Posted: Aug 05, 2018 16:25 Post subject: Re: Collection Macros |
|
|
these are separate examples. One of my apparent Fluorite specimens dissolved hard in dilute muriatic acid. It was a definite cube, which leads me to wonder if Fluorite cannot be dissolved by that solution or if calcite can grow in cubic crystal structure? the original rhombic shaped crystals are in fact fluorite, no fizz from vinegar, passed hardness tests. I will post more pictures now.
| Mineral: | Fluorite |
| Locality: | | Alum Creek, Columbus, Franklin County, Ohio, USA |  |
|
| Dimensions: | 1cm x 1cm (Crystal) |
| Description: |
|
| Viewed: |
15414 Time(s) |

|
| Mineral: | Fluorite |
| Locality: | | Alum Creek, Columbus, Franklin County, Ohio, USA |  |
|
| Dimensions: | 1cm x 1cm (Crystal) |
| Description: |
|
| Viewed: |
15384 Time(s) |

|
| Mineral: | Fluorite |
| Locality: | | Alum Creek, Columbus, Franklin County, Ohio, USA |  |
|
| Dimensions: | 1cm x 1cm (Crystal) |
| Description: |
|
| Viewed: |
15448 Time(s) |

|
|
|
| Back to top |
|
 |
Bob Harman
Joined: 06 Nov 2015
Posts: 765



|
 Posted: Aug 05, 2018 16:57 Post subject: Re: Collection Macros Posted: Aug 05, 2018 16:57 Post subject: Re: Collection Macros |
|
|
In my opinion, all that you are showing are small crystals and cleavage portions of calcite. I see no fluorite! If your other example dissolved in dilute muriatic acid it also was calcite. That acid (28% HCl) specifically dissolves carbonates (including calcite) while having no effect on most non-carbonates including fluorite.
In addition.....and this can be important.....if all your specimens came from fill along a bike path along Alum Creek in Columbus Ohio, it would be statically much more likely to find pieces of calcite rather than fluorite. Calcite veins and small crystals are very common in nearby limestone, so pieces of calcite in your examples would be common while fluorite pieces would be comparatively quite uncommon making it unlikely that you found any.
OLIVER, since you seem to be enthusiastic, I suggest you join a local club and have the experienced club members help get you going. BOB
|
|
| Back to top |
|
 |
Tom Tucker
Joined: 03 Dec 2009
Posts: 60
Location: Virginia


|
 Posted: Aug 05, 2018 17:01 Post subject: Re: Collection Macros Posted: Aug 05, 2018 17:01 Post subject: Re: Collection Macros |
|
|
| Your three newly posted photos show calcite only. Tom
|
|
| Back to top |
|
 |
Jim Robison
Joined: 17 Nov 2010
Posts: 55


|
 Posted: Aug 05, 2018 17:33 Post subject: Re: Collection Macros Posted: Aug 05, 2018 17:33 Post subject: Re: Collection Macros |
|
|
An important clue is the difference in shape between the two minerals. Fluorite is aa cubic mineral, which among other things mean the corners of natural crystals have a square shape or a variation on the same. Calcite is in a different crystal system, and has rhombohedral faces that are all inclined. In both minerals there are sets of parallel opposite faces, but in calcite they are inclined from the square. Fluorite always has some sets of parallel faces that are opposite but of a different orientation, but always based on the cubic oraientation. . You need to look at crystal shapes in pictures and at information on crystal shapes. I can't remember where on the Forum you find that, but believe there is a section on this topic.
As your collecting advances you will quickly discover that the form of a crystal, or a cleavage shape, is needed.
|
|
| Back to top |
|
 |
Oliver B
Joined: 30 Jul 2018
Posts: 27
Location: Columbus, Ohio


|
 Posted: Aug 05, 2018 18:13 Post subject: Re: Collection Macros Posted: Aug 05, 2018 18:13 Post subject: Re: Collection Macros |
|
|
| thanks all, there isnt much to find here in columbus and i havent found any clubs or organizations locally that i can join but ill keep learning the best i can!
|
|
| Back to top |
|
 |
|





