| View previous topic :: View next topic |
| Author |
Message |
Pete Richards
Site Admin

Joined: 29 Dec 2008
Posts: 846
Location: Northeast Ohio



|
 Posted: Nov 28, 2023 08:35 Post subject: Re: Fluorite crystallography? Posted: Nov 28, 2023 08:35 Post subject: Re: Fluorite crystallography? |
|
|
| Carles Millan wrote: | | So it is {100}{110}. |
Yes, it is {100}{110}. I checked my small collection of Naica fluorites last night, and mine also have this habit.
An interesting extra is that some of them have an overall octahedral shape, like ones that Peter Megaw illustrated. In spite of the overall shape, the faces on the specimen are all cube or dodecahedral faces. It seems quite likely that there was a first generation that was octahedral, and the second generation of cube and dodecahedron overgrew it. I'll add a photo if I can get a decent one....
_________________
Collecting and studying crystals with interesting habits, twinning, and epitaxy |
|
| Back to top |
|
 |
Tobi
Site Admin

Joined: 07 Apr 2009
Posts: 4255
Location: Germany



|
 Posted: Nov 28, 2023 10:00 Post subject: Re: Fluorite crystallography? Posted: Nov 28, 2023 10:00 Post subject: Re: Fluorite crystallography? |
|
|
| Tobi wrote: | | Peter Megaw wrote: | | Tobi...could I get high-rez versions of these images with permission to publish? I am working on an article on Naica and these are worth considering for inclusion. |
I'll let you know if a larger (and better) version is possible ;-) |
Hi Peter, I just received an answer from Rudi Watzl's assistant Konrad, he sent me the largest version of the file that they have. Resolution is the same but the file size is much larger, I hope this image that I attached to this post is good enough for you?
Concerning the credits he wrote:
"Natürlich könnt ihr das Foto gerne auch veröffentlichen. Bitte bei den Credits „Foto: Saphira Minerals“ schreiben." ("Of course you are welcome to publish the photo. Please write “Photo: Saphira Minerals” in the credits.")
So please feel free to use it :-)
Regards
Tobi
| Mineral: | Fluorite, Sphalerite |
| Locality: | | Naica Mine, Naica, Municipio Saucillo, Chihuahua, Mexico |  |
|
| Dimensions: | Specimen height 8 cm |
| Description: |
|
| Viewed: |
90235 Time(s) |

|
|
|
| Back to top |
|
 |
Jesse Fisher

Joined: 18 Mar 2009
Posts: 641
Location: San Francisco



|
 Posted: Nov 28, 2023 11:08 Post subject: Re: Fluorite crystallography? Posted: Nov 28, 2023 11:08 Post subject: Re: Fluorite crystallography? |
|
|
Another Naica fluorite, which according to Pete's analysis would be cube-dodechahedral habit. From the find circa 2005.
| Mineral: | Fluorite on Sphalerite |
| Locality: | | Naica Mine, Naica, Municipio Saucillo, Chihuahua, Mexico |  |
|
| Dimensions: | 14x10x6 cm |
| Description: |
| cube-dodecahedral habit, with inclusions of what appears to be pyrite. |
|
| Viewed: |
90174 Time(s) |

|
|
|
| Back to top |
|
 |
Bob Morgan
Joined: 18 Jan 2018
Posts: 254
Location: Savannah, Georgia



|
 Posted: Nov 28, 2023 11:45 Post subject: Re: Fluorite crystallography? Posted: Nov 28, 2023 11:45 Post subject: Re: Fluorite crystallography? |
|
|
Pete,
Some of us call these 'Mayan Temple" habit fluorites. Yes, these are the result of smaller overgrowths on octahedral crystals. I have a specimen from Naica that has an octahedral phantom sprinkled with small galena crystals, that can be seen through clear cube faces at the terminations of the underlying octahedron. This particular specimen has overgrowths that are cubes with small dodecahedral faces and larger trapezohedral faces and the occasional small octahedral face.
The original octahedral stage is an indication of a higher temperature of formation, which is supported by the octahedral habit of the small galena crystals. Subsequent overgrowths then were lower temperature aligned deposits more characteristic of cube shapes.
'Myan Temple' habit fluorites come from other places. I've seen them from Dalnegorsk, China and even places in the US that I can't recall.
Larger overgrowths tend to be on the octahedral terminations with smaller ones down the octahedral edges and even smaller ones stepping all over underlying octahedral faces.
|
|
| Back to top |
|
 |
Pete Richards
Site Admin

Joined: 29 Dec 2008
Posts: 846
Location: Northeast Ohio



|
 Posted: Nov 28, 2023 13:40 Post subject: Re: Fluorite crystallography? Posted: Nov 28, 2023 13:40 Post subject: Re: Fluorite crystallography? |
|
|
| Bob Morgan wrote: | (snip)
Larger overgrowths tend to be on the octahedral terminations with smaller ones down the octahedral edges and even smaller ones stepping all over underlying octahedral faces. |
Bob's comments about overgrowth patterns are supported by the photos below of one of my Naica specimens. Peter Megaw's photos earlier also show the general pattern of overgrowth on an earlier octahedron - particularly the second one in which the overgrowth is only minimal.
The slower and somewhat chaotic overgrowth in the middles of the original octahedral faces leaves them depressed in comparison to the corners and edges adorned by the cube and dodecahedral faces.
| Mineral: | Fluorite |
| Locality: | | Naica, Municipio Saucillo, Chihuahua, Mexico |  |
|
| Dimensions: | 2 cm |
| Description: |
| View showing cube and dodecahedal overgrowths on a corner and edges of a former octahedral crystal. Natural light. |
|
| Viewed: |
90081 Time(s) |

|
| Description: |
|
| Viewed: |
90259 Time(s) |

|
| Mineral: | Fluorite |
| Locality: | | Naica, Municipio Saucillo, Chihuahua, Mexico |  |
|
| Description: |
| Top view with cube face highlighted. Artificial light. |
|
| Viewed: |
90120 Time(s) |

|
_________________
Collecting and studying crystals with interesting habits, twinning, and epitaxy |
|
| Back to top |
|
 |
Bob Morgan
Joined: 18 Jan 2018
Posts: 254
Location: Savannah, Georgia



|
 Posted: Dec 04, 2023 12:19 Post subject: Re: Fluorite crystallography? Posted: Dec 04, 2023 12:19 Post subject: Re: Fluorite crystallography? |
|
|
| The picture you feature under your name is cube-octahedral.
|
|
| Back to top |
|
 |
Bob Morgan
Joined: 18 Jan 2018
Posts: 254
Location: Savannah, Georgia



|
 Posted: Dec 04, 2023 12:23 Post subject: Re: Fluorite crystallography? Posted: Dec 04, 2023 12:23 Post subject: Re: Fluorite crystallography? |
|
|
| The picture I referenced is under Carles Millan's name.
|
|
| Back to top |
|
 |
Johan Kjellman

Joined: 10 Jan 2014
Posts: 19
Location: Uppsala


|
 Posted: Dec 12, 2023 17:58 Post subject: Re: Fluorite crystallography? Posted: Dec 12, 2023 17:58 Post subject: Re: Fluorite crystallography? |
|
|
| Jesse Fisher wrote: | | Another Naica fluorite, which according to Pete's analysis would be cube-dodechahedral habit. From the find circa 2005. |
I 100 % agree with Pete's analysis of how to differentiate between the two combinations.
But in the case of this (Jesse's) fluorite it appears to me to be a cube-octahedron.
cheers
|
|
| Back to top |
|
 |
Bob Morgan
Joined: 18 Jan 2018
Posts: 254
Location: Savannah, Georgia



|
 Posted: Dec 12, 2023 18:03 Post subject: Re: Fluorite crystallography? Posted: Dec 12, 2023 18:03 Post subject: Re: Fluorite crystallography? |
|
|
| Yes.
|
|
| Back to top |
|
 |
Pete Richards
Site Admin

Joined: 29 Dec 2008
Posts: 846
Location: Northeast Ohio



|
 Posted: Dec 12, 2023 20:52 Post subject: Re: Fluorite crystallography? Posted: Dec 12, 2023 20:52 Post subject: Re: Fluorite crystallography? |
|
|
I am not sure how to interpret Jesse's fluorite. I cannot see any view that is unambiguous. It appears that the cube faces are clear, maybe slightly pebbled, and that the other faces are frosted.
In the bottom-most fluorite in his specimen, the lower left corner of the forward-facing cube face points to the edge between a pair of the other faces, beyond which is a face that is just barely visible because it is nearly perpendicular to the view. This face holds the answer. If it is a cube face, then the habit is cube-octahedral; if it is one of the other, frosted faces, the habit is cube-dodecahedral.
The crystal on the upper left also helps a little. It has a cube face oriented forward and slightly down. Above and to the right of this face are two of the other faces. To the top-right of these is another face, sloping backwards. It is hard to see clearly, but it appears to me to be frosted. If so, this crystal also indicates a cube-dodecahedral habit; if instead this is a cube face, then the habit is cube-octahedral.
In my view, and with considerable uncertainty, the habit of this one is, like the other examples, cube-dodecahedral - (100)(110). Perhaps Jesse will look at his specimen from all angles and let us know!
_________________
Collecting and studying crystals with interesting habits, twinning, and epitaxy |
|
| Back to top |
|
 |
Bob Morgan
Joined: 18 Jan 2018
Posts: 254
Location: Savannah, Georgia



|
 Posted: Dec 12, 2023 22:29 Post subject: Re: Fluorite crystallography? Posted: Dec 12, 2023 22:29 Post subject: Re: Fluorite crystallography? |
|
|
Pete,
Points well stated.
I looked at photos in Mindat of fluorite on sphalerite. Nearly all were cube- dodecahedral that could be discerned clearly.
|
|
| Back to top |
|
 |
Johan Kjellman

Joined: 10 Jan 2014
Posts: 19
Location: Uppsala


|
 Posted: Dec 13, 2023 02:57 Post subject: Re: Fluorite crystallography? Posted: Dec 13, 2023 02:57 Post subject: Re: Fluorite crystallography? |
|
|
I agree again - and withdraw my claim.
And Pete, you point directly at what I now think so many of us, although experienced, keep on misinterpreting these combos. We are all fast and confident in identifying the first prominent cube face. Then a "second face" a bit too quickly identified as a cube and, voila, you have a cube-octahedron.
Maybe we all should slow down and look for a third alternative cube face. I (think I) see them in both crystals you described on Jesse's specimen.The thing is, and this may be part of the problem, these cube faces seem relatively smaller than the obvious large one.
cheers
|
|
| Back to top |
|
 |
Pete Richards
Site Admin

Joined: 29 Dec 2008
Posts: 846
Location: Northeast Ohio



|
 Posted: Dec 13, 2023 09:42 Post subject: Re: Fluorite crystallography? Posted: Dec 13, 2023 09:42 Post subject: Re: Fluorite crystallography? |
|
|
| Johan Kjellman wrote: | (snip)
The thing is, and this may be part of the problem, these cube faces seem relatively smaller than the obvious large one. |
Yes, definitely. These crystals show substantial deviations from ideally developed morphology, where all faces of a given form would have the same size and shape. Several of the dodecahedral faces appear to have nice three-fold symmetry, which is a false impression, but possible since these faces have six edges. With a little distortion, they can present as alternating long and short edges, and voila, pseudo-three-fold symmetry. The angles between the edges would be wrong for three-fold symmetry, but this is very hard to judge when a face is tilted.
_________________
Collecting and studying crystals with interesting habits, twinning, and epitaxy |
|
| Back to top |
|
 |
Johan Kjellman

Joined: 10 Jan 2014
Posts: 19
Location: Uppsala


|
 Posted: Oct 01, 2024 05:15 Post subject: Re: Fluorite crystallography? Posted: Oct 01, 2024 05:15 Post subject: Re: Fluorite crystallography? |
|
|
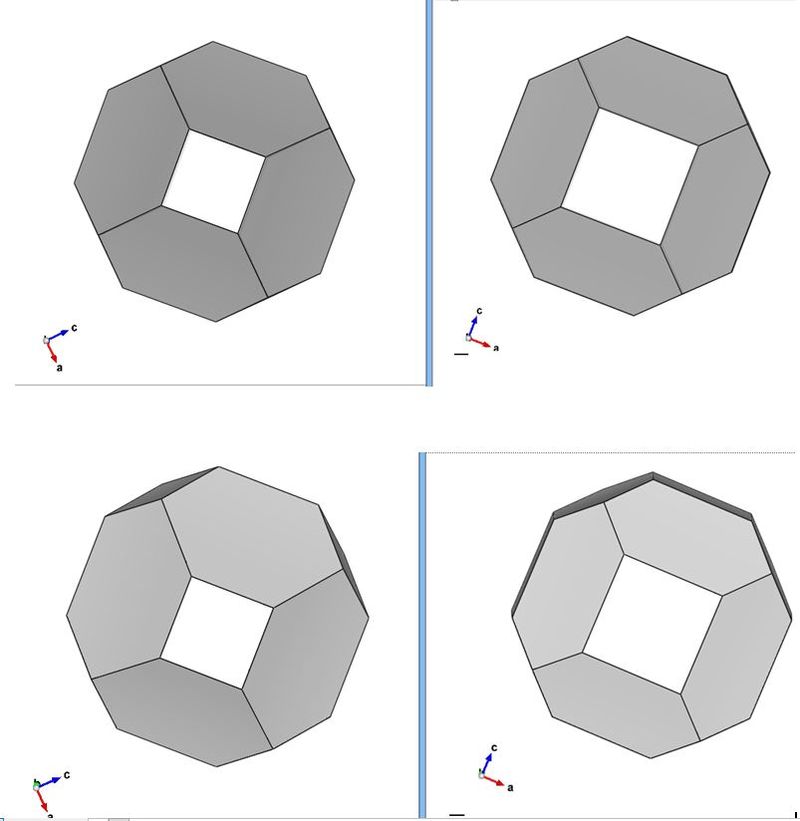
I came back to this thread, having forgotten and identified the first image first as a cube-octahedron, but then, after counting the exposed faces of the most obvious crystal (I could see 8) I came to the conclusion that it must be a cube-dodecahedron. Because you can never see more faces than half of what the crystal have from one side. I am quite confident that this applies even to distorted crystals, even stepped crystals if you count repeatedly oriented faces as 1.
So, I propose the following "half-face" method, not bulletproof, but works in many instances.
cube + octahedron = 6 + 8 faces = 14, one should not be able to see more than 7 faces
cube + dodecahedron = 6 + 12 faces = 18, i.e. not more than 9
I attach a hastily prepared sketch. The top two crystals are different but oriented in a way that minimize the number of the exposed faces. This illustrates the general problem.
On the bottom row I have tilted both crystals, and you can now count the exposed faces.
| Description: |
|
| Viewed: |
18212 Time(s) |
|
|
|
| Back to top |
|
 |
|





