| View previous topic :: View next topic |
| Author |
Message |
Jlea80

Joined: 19 Oct 2019
Posts: 8
Location: Arkansas


|
 Posted: Nov 15, 2020 04:24 Post subject: What is this? Posted: Nov 15, 2020 04:24 Post subject: What is this? |
|
|
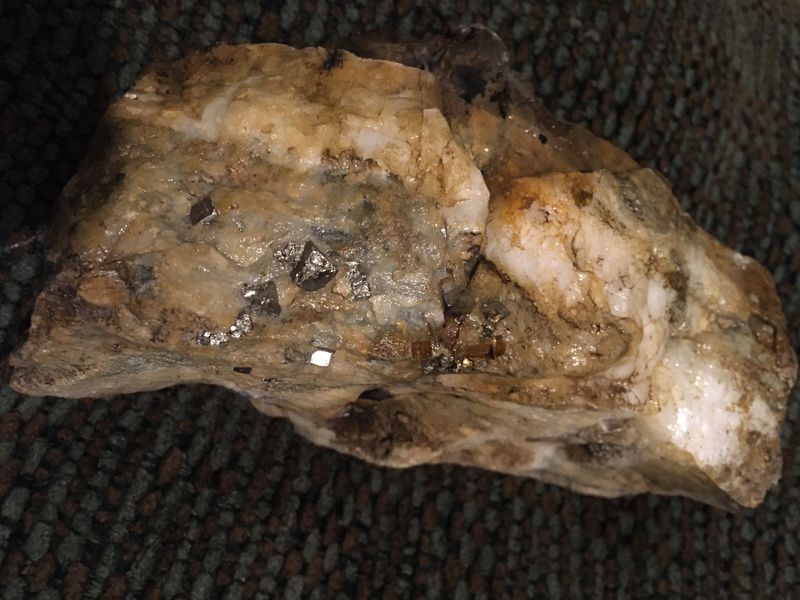
This belonged to my great great uncle, he was an avid collector and had rocks from around the world so I can’t tell you the locality that this particular rock came from but I’m going to try my best on describing everything else.
These tiny, Very shiny, Goldish/Bronze rhomboid 3 dimensional Hard objects are protruding out of a layered quartz rock. Although the top is very dull, lumpy , porous, and the top is also various shades of brown earth tones from creamy to rustic . I put a drop of that acid stuff on it, no reaction at all. It’s very heavy.
| Mineral: | Quartz |
| Dimensions: | 50cm |
| Description: |
|
| Viewed: |
10606 Time(s) |
|
| Mineral: | Quartz |
| Dimensions: | 50cm |
| Description: |
|
| Viewed: |
10597 Time(s) |

|
| Mineral: | Quartz |
| Dimensions: | 50cm |
| Description: |
|
| Viewed: |
10601 Time(s) |

|
|
|
| Back to top |
|
 |
Josele

Joined: 10 Apr 2012
Posts: 410
Location: Tarifa, Spain



|
 Posted: Nov 15, 2020 05:18 Post subject: Re: What is this? Posted: Nov 15, 2020 05:18 Post subject: Re: What is this? |
|
|
| Looks like pyrite on a quartz vein in a kind of metamorphic rock.
|
|
| Back to top |
|
 |
Sante Celiberti
Joined: 04 Oct 2019
Posts: 699
Location: Tuscany



|
 Posted: Nov 15, 2020 06:05 Post subject: Re: What is this? Posted: Nov 15, 2020 06:05 Post subject: Re: What is this? |
|
|
I agree with Josele.
Definitely unmistakable.
|
|
| Back to top |
|
 |
Jlea80

Joined: 19 Oct 2019
Posts: 8
Location: Arkansas


|
 Posted: Nov 15, 2020 06:22 Post subject: Re: What is this? Posted: Nov 15, 2020 06:22 Post subject: Re: What is this? |
|
|
| Thank you both for the replies. It’s definitely an original 😊 ..So pyrite has no reaction to the acid test? And if I cut the rock in half what do you think would be inside?
|
|
| Back to top |
|
 |
Sante Celiberti
Joined: 04 Oct 2019
Posts: 699
Location: Tuscany



|
 Posted: Nov 15, 2020 07:45 Post subject: Re: What is this? Posted: Nov 15, 2020 07:45 Post subject: Re: What is this? |
|
|
Carbonates only react to sulphuric acid, although in different ways. Pyrite, being a sulphide, doesn't.
If you cut your specimen you could very probably find the same cubic crystals of pyrite, although hardly they could be intact, due to the breaking shock and strong aderence to the quartzitic host matrix. Unless you are so fortunate to find, in such a large piece, a small cavity with intact crystals. But I doubt this could be the case.
Good luck.
Sante
|
|
| Back to top |
|
 |
Peter Perkins

Joined: 17 Nov 2012
Posts: 87
Location: Norfolk,UK


|
 Posted: Nov 15, 2020 08:21 Post subject: Re: What is this? Posted: Nov 15, 2020 08:21 Post subject: Re: What is this? |
|
|
| I think it would be more rewarding to break it in half, rather than cut it. The freshly broken surfaces might show cubes of Pyrite sticking out. If it was cut, there would be patches of gold colour where the cubes were located. If broken you might well be able to tease a cube out of the rock.
|
|
| Back to top |
|
 |
Josele

Joined: 10 Apr 2012
Posts: 410
Location: Tarifa, Spain



|
 Posted: Nov 15, 2020 09:56 Post subject: Re: What is this? Posted: Nov 15, 2020 09:56 Post subject: Re: What is this? |
|
|
| Sante Celiberti wrote: | | Carbonates only react to sulphuric acid, although in different ways. Pyrite, being a sulphide, doesn't. ... |
Sante, i suppose you mean chlorhydric acid, isn't it?
I never checked the reaction of carbonates with sulphuric, maybe it works too?
|
|
| Back to top |
|
 |
lluis
Joined: 17 Nov 2006
Posts: 719


|
 Posted: Nov 15, 2020 10:14 Post subject: Re: What is this? Posted: Nov 15, 2020 10:14 Post subject: Re: What is this? |
|
|
Carbonates react with acids, mineral or organic. With some quicker, with some slower.
Chlorhidirc acid is better for attack because many of chlorides are soluble and they do not make a interlayer that stops attack. Which would be the case in cerusite (lead chloride is insoluble at room temperature, so, attack would be very ralentized....)
Attack with nitric acid would be surer (I do not know any insoluble nitrate (it may exist, though...). But nitric acid is corrosive to skin, far more than chlorhidric.,...
With sulphuric acid, calcite would be covered with gypsum (hydrated calcium sulphate, and attack would be also relented...)
And, by the way, sulphides are also attacked by acids. Mainly by chlorhidric acid.. (for one side, chlorhydric acid is stronger than sulphidric acid,so, it displaces it, and on another side, chlorides are an excellent complexant agent, so, they ease the attack to cations...
Pyrites are attacked by chlorhidric acid... Maybe not very quick, but let a piece of pyrite that you do not like in a recipient with water and some chlorhidric acid,,,,, You would get rust... and probably a disagreeable rotten eggs smell.....
With best wishes
Lluís
|
|
| Back to top |
|
 |
Sante Celiberti
Joined: 04 Oct 2019
Posts: 699
Location: Tuscany



|
 Posted: Nov 15, 2020 15:40 Post subject: Re: What is this? Posted: Nov 15, 2020 15:40 Post subject: Re: What is this? |
|
|
| Josele wrote: | | Sante Celiberti wrote: | | Carbonates only react to sulphuric acid, although in different ways. Pyrite, being a sulphide, doesn't. ... |
Sante, i suppose you mean chlorhydric acid, isn't it?
I never checked the reaction of carbonates with sulphuric, maybe it works too? |
Hello, Josele.
Of course, I meant chlorhydric. But yesterday I was helping my grandson with his schoolwork on sulphuric acid, since my mistake.
Stay healthy.
|
|
| Back to top |
|
 |
Carles Millan
Site Admin

Joined: 05 May 2007
Posts: 1538
Location: Catalonia



|
 Posted: Nov 15, 2020 16:31 Post subject: Re: What is this? Posted: Nov 15, 2020 16:31 Post subject: Re: What is this? |
|
|
For the sake of clarity for English speakers, I would like to point out that chlorhydric acid actually refers to hydrochloric acid.
https://en.wikipedia.org/wiki/Hydrochloric_acid
|
|
| Back to top |
|
 |
Sante Celiberti
Joined: 04 Oct 2019
Posts: 699
Location: Tuscany



|
 Posted: Nov 15, 2020 16:43 Post subject: Re: What is this? Posted: Nov 15, 2020 16:43 Post subject: Re: What is this? |
|
|
| lluis wrote: | Carbonates react with acids, mineral or organic. With some quicker, with some slower.
Chlorhidirc acid is better for attack because many of chlorides are soluble and they do not make a interlayer that stops attack. Which would be the case in cerusite (lead chloride is insoluble at room temperature, so, attack would be very ralentized....)
Attack with nitric acid would be surer (I do not know any insoluble nitrate (it may exist, though...). But nitric acid is corrosive to skin, far more than chlorhidric.,...
With sulphuric acid, calcite would be covered with gypsum (hydrated calcium sulphate, and attack would be also relented...)
And, by the way, sulphides are also attacked by acids. Mainly by chlorhidric acid.. (for one side, chlorhydric acid is stronger than sulphidric acid,so, it displaces it, and on another side, chlorides are an excellent complexant agent, so, they ease the attack to cations...
Pyrites are attacked by chlorhidric acid... Maybe not very quick, but let a piece of pyrite that you do not like in a recipient with water and some chlorhidric acid,,,,, You would get rust... and probably a disagreeable rotten eggs smell.....
With best wishes
Lluís |
Thank you, Lluis, for your instructive informations.
Knowledge of a chemist is always appreciated, as well as helpful. I learn a lot from your posts.
I know that the statement "sulphides don't react..." is scientifically uncorrect but, since in collector's practice the "acid test" is mainly referred to effervescent reaction in carbonates (absent in sulphides), this is the concept I wanted to transfer to the novice Jlea80.
I hope you agree on an educational dosage of knowledge. :-)
Warm greetings.
|
|
| Back to top |
|
 |
Jlea80

Joined: 19 Oct 2019
Posts: 8
Location: Arkansas


|
 Posted: Nov 15, 2020 22:20 Post subject: Re: What is this? Posted: Nov 15, 2020 22:20 Post subject: Re: What is this? |
|
|
| Wow! I’m very impressed with you guys. Thank you for making me feel comfortable. This was fun and I’ve learned So much! ... I think I’m going to break the rock soon! 😊
|
|
| Back to top |
|
 |
|





