| View previous topic :: View next topic |
| Author |
Message |
Daniel Garcia
Joined: 02 May 2022
Posts: 23
Location: Narbonne


|
 Posted: Apr 08, 2025 03:34 Post subject: cristobalite vs chalcedony in thin section Posted: Apr 08, 2025 03:34 Post subject: cristobalite vs chalcedony in thin section |
|
|
Dear all
I'm trying to document a silicification event in Oligocene evaporites near Narbonne (France)
The host rock is gypsiferous, with some diagenetic native sulfur. Silicification occurs sporadically, either as thin layers intercalated with clays and microcystalline gypsum (algal laminae ?) or as dispersed spherulites with a distinct zoning pattern. My question is how much cristobalite and how much chalcedony in these assemblages ?
| Mineral: | cristobalite |
| Dimensions: | 1 x 2 mm |
| Description: |
| PPL image. Spherulites in gypsum sandy layer |
|
| Viewed: |
21604 Time(s) |

|
|
|
| Back to top |
|
 |
marco campos-venuti

Joined: 09 Apr 2014
Posts: 240
Location: Sevilla



|
 Posted: Apr 08, 2025 03:40 Post subject: Re: cristobalite vs chalcedony in thin section Posted: Apr 08, 2025 03:40 Post subject: Re: cristobalite vs chalcedony in thin section |
|
|
| Cristobalite is high T mineral. All supposed cristobalite spherulites are other silica phase.
|
|
| Back to top |
|
 |
Daniel Garcia
Joined: 02 May 2022
Posts: 23
Location: Narbonne


|
 Posted: Apr 08, 2025 04:07 Post subject: Re: cristobalite vs chalcedony in thin section Posted: Apr 08, 2025 04:07 Post subject: Re: cristobalite vs chalcedony in thin section |
|
|
| marco campos-venuti wrote: | | Cristobalite is high T mineral. All supposed cristobalite spherulites are other silica phase. |
So, no way to identify the textural pattern ?
Here is a macro view of the spherulites
| Mineral: | cristobalite |
| Dimensions: | 0.5 x 1 cm |
| Description: |
|
| Viewed: |
21593 Time(s) |

|
|
|
| Back to top |
|
 |
marco campos-venuti

Joined: 09 Apr 2014
Posts: 240
Location: Sevilla



|
 Posted: Apr 08, 2025 05:38 Post subject: Re: cristobalite vs chalcedony in thin section Posted: Apr 08, 2025 05:38 Post subject: Re: cristobalite vs chalcedony in thin section |
|
|
| My impression is that it is all chalcedony. Chalcedony is a fibrous silica polymer so the structure you see in thin section looks very similar to it. Cristobalite is stable over 1470ºC, Therefore it is impossible to find it in a diagenetic environment. I have written several books about the equilibrium of microcrystalline silica phases that you can see on my website.
|
|
| Back to top |
|
 |
Daniel Garcia
Joined: 02 May 2022
Posts: 23
Location: Narbonne


|
 Posted: Apr 08, 2025 06:53 Post subject: Re: cristobalite vs chalcedony in thin section Posted: Apr 08, 2025 06:53 Post subject: Re: cristobalite vs chalcedony in thin section |
|
|
| marco campos-venuti wrote: | | My impression is that it is all chalcedony. Chalcedony is a fibrous silica polymer so the structure you see in thin section looks very similar to it. Cristobalite is stable over 1470 C, Therefore it is impossible to find it in a diagenetic environment. I have written several books about the equilibrium of microcrystalline silica phases that you can see on my website. |
Hi Marco, thanks. Of course, I don't claim cristobalite to be a stable phase here.
I just try to understand what controls the intergrowth pattern of the fibers, Here is another view of this texture
| Mineral: | cristobalite |
| Dimensions: | 0.5 x 1 mm |
| Description: |
| PPL view microcristalline silica replacing gypsum |
|
| Viewed: |
21562 Time(s) |

|
| Mineral: | cristobalite |
| Dimensions: | 0.5 x 1 mm |
| Description: |
| XPL view microcystalline silica replacing gypsum |
|
| Viewed: |
21559 Time(s) |

|
|
|
| Back to top |
|
 |
marco campos-venuti

Joined: 09 Apr 2014
Posts: 240
Location: Sevilla



|
 Posted: Apr 08, 2025 07:59 Post subject: Re: cristobalite vs chalcedony in thin section Posted: Apr 08, 2025 07:59 Post subject: Re: cristobalite vs chalcedony in thin section |
|
|
| I don't see replacing after gypsum. The formation of your spherulites looks like the growth of a concretion. There is a granular gypsum with porosity. A Si-rich solution flows through the porosity. The solution dehydrates and polymerizes. Monomeric Si ions become dimers, then trimers, etc. When the ions reach 1 nm in diameter we have a colloidal solution that behaves like a hydrogel. When this dehydrates further, it begins to crystallize the chalcedony which is fibrous. Chalcedony is the stable phase in an acidic environment, if the conditions are basic we have opal. New space is created on the external surface of the chalcedony by dissolution. Little by little one band at a time the spherulite grows. Compared to the crystallization of quartz crystals in gypsum Keuper deposits, in your case the climate must have been hot and dry. Without a doubt we are in subaerial conditions because the hydrogel is water-soluble and does not form under water.
|
|
| Back to top |
|
 |
Peter Megaw
Site Admin

Joined: 13 Jan 2007
Posts: 975
Location: Tucson, Arizona



|
 Posted: Apr 08, 2025 09:08 Post subject: Re: cristobalite vs chalcedony in thin section Posted: Apr 08, 2025 09:08 Post subject: Re: cristobalite vs chalcedony in thin section |
|
|
Robert Folk taught that length-slow chalcedony was indicative of formation under evaporitic or sulfate rich conditions. If you have a gyp plate you could check that out
_________________
Siempre Adelante! |
|
| Back to top |
|
 |
Roger Warin

Joined: 23 Jan 2013
Posts: 1250



|
 Posted: Apr 08, 2025 09:14 Post subject: Re: cristobalite vs chalcedony in thin section Posted: Apr 08, 2025 09:14 Post subject: Re: cristobalite vs chalcedony in thin section |
|
|
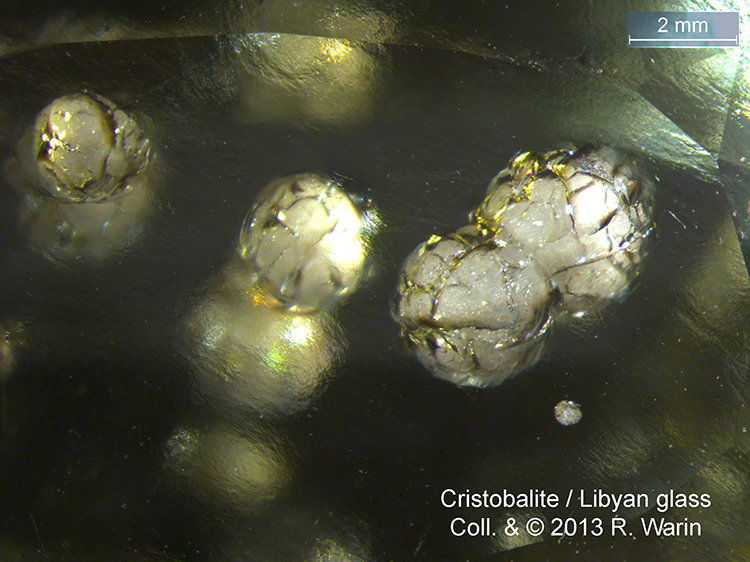
As I observed in LDG, cristobalite microcrystals readily aggregate into micro-ourses that occupy an intercalated phase (spherules) in another phase, as in semi-crystalline polymers.
I insist to my friend Herwig that I observe a Maltese cross in my case for Libyan glass (which has never been said before for LDG).
| Mineral: | Cristobalite |
| Description: |
| Locality: Egypt - Libian Desert |
|
| Viewed: |
21505 Time(s) |
|
| Mineral: | Cristobalite |
| Description: |
|
| Viewed: |
21510 Time(s) |

|
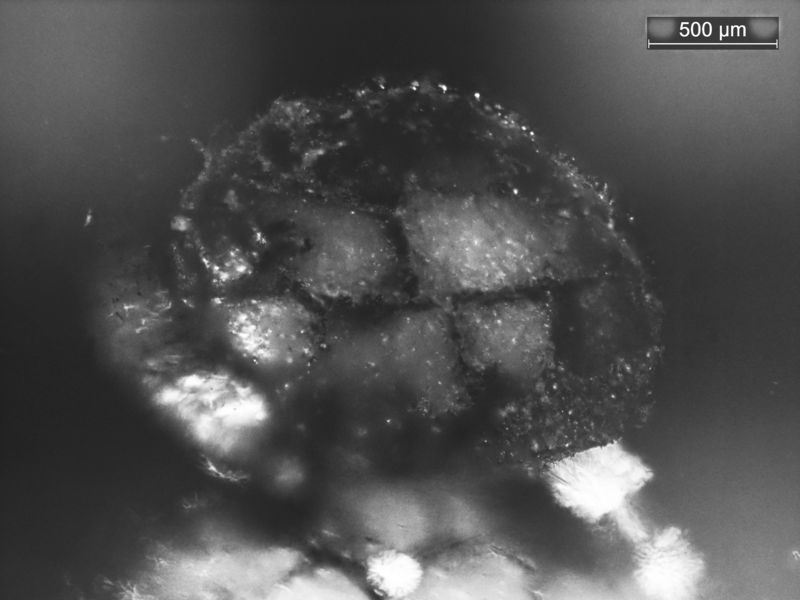
| Mineral: | Cristobalite |
| Description: |
|
| Viewed: |
21502 Time(s) |
|
|
|
| Back to top |
|
 |
Daniel Garcia
Joined: 02 May 2022
Posts: 23
Location: Narbonne


|
 Posted: Apr 08, 2025 09:27 Post subject: Re: cristobalite vs chalcedony in thin section Posted: Apr 08, 2025 09:27 Post subject: Re: cristobalite vs chalcedony in thin section |
|
|
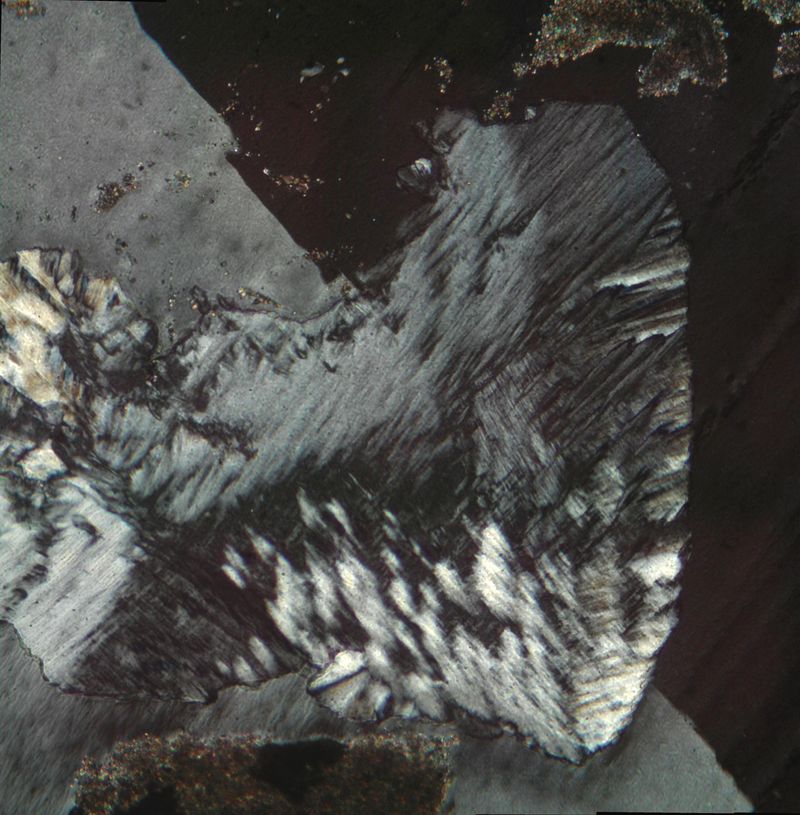
Sorry to be unclear. What I see is that silica (chalcedony) takes the place of former gypsum lenses, growing from the faces inwards. I agree that the likely mechanisme is coupled dissolution-precipitation. Maybe replacement was not the proper term.
But my main question remains. Can we derive any inference from the treillis structure of this (chalcedony) intergrowth ? It cannot be true HT cristobalite, but at least it displays a symetry that is reminiscent of an isometric or pseudoisometric frame. Does this mean there was an isometric or amorphous precursor ?
Thanks anyway for replying
| Mineral: | chalcedony |
| Dimensions: | 0.5 x 1 mm |
| Description: |
| XPL treillis structure in chalcedony (?), growing across a gypsum twin plane |
|
| Viewed: |
21502 Time(s) |
|
|
|
| Back to top |
|
 |
Daniel Garcia
Joined: 02 May 2022
Posts: 23
Location: Narbonne


|
 Posted: Apr 08, 2025 09:34 Post subject: Re: cristobalite vs chalcedony in thin section Posted: Apr 08, 2025 09:34 Post subject: Re: cristobalite vs chalcedony in thin section |
|
|
| Peter Megaw wrote: | | Robert Folk taught that length-slow chalcedony was indicative of formation under evaporitic or sulfate rich conditions. If you have a gyp plate you could check that out |
Thanks, Yes I did.
Unfortunately the fibers you see in all the preceeding pictures do not have a straight extinction, hence defining an optical sign is problematic.
On the other hand, I do see in the same thin section more conventional chalcedony fibers that do have a straight extinction and a positive sign (leght slow, like quartz)
|
|
| Back to top |
|
 |
marco campos-venuti

Joined: 09 Apr 2014
Posts: 240
Location: Sevilla



|
 Posted: Apr 08, 2025 10:16 Post subject: Re: cristobalite vs chalcedony in thin section Posted: Apr 08, 2025 10:16 Post subject: Re: cristobalite vs chalcedony in thin section |
|
|
Hi Daniel,
From the hand sample the chalcedony is spherical and I don't see any faces. From the last photo you can see chalcedony filling a cavity inside a gypsum crystal, but I still don't see any faces.
If you are looking for a replacement, the original crystal must be in disequilibrium and in your case the gypsum is not in disequilibrium so you don't have a replacement of gypsum, but only filling a cavity in the gypsum. It could also be a primary cavity of the gypsum or a grain included in the gypsum and then dissolved.
However, an evaporite is an alkaline environment and therefore it would be good for CT-opal to precipitate which is amorphous and is often confused with cristobalite, as in the case of Roger's LDG.. Then the opal is replaced by chalcedony later when the chemistry becomes acidic.
|
|
| Back to top |
|
 |
Daniel Garcia
Joined: 02 May 2022
Posts: 23
Location: Narbonne


|
 Posted: Apr 08, 2025 11:13 Post subject: Re: cristobalite vs chalcedony in thin section Posted: Apr 08, 2025 11:13 Post subject: Re: cristobalite vs chalcedony in thin section |
|
|
| marco campos-venuti wrote: | Hi Daniel,
From the hand sample the chalcedony is spherical and I don't see any faces. From the last photo you can see chalcedony filling a cavity inside a gypsum crystal, but I still don't see any faces.
If you are looking for a replacement, the original crystal must be in disequilibrium and in your case the gypsum is not in disequilibrium so you don't have a replacement of gypsum, but only filling a cavity in the gypsum. It could also be a primary cavity of the gypsum or a grain included in the gypsum and then dissolved.
However, an evaporite is an alkaline environment and therefore it would be good for CT-opal to precipitate which is amorphous and is often confused with cristobalite, as in the case of Roger's LDG.. Then the opal is replaced by chalcedony later when the chemistry becomes acidic. |
Hence you suggest a CT-opal precursor ?
And the chalcedony inheriting some of the short range organization of its precursor ?
Concerning the question of gypsum replacement vs silica filling a former cavity, I will not argue further on, the question seems a bit academic. I was'nt there when it occured.
|
|
| Back to top |
|
 |
alfredo
Site Admin

Joined: 30 Jan 2008
Posts: 1018



|
 Posted: Apr 08, 2025 21:34 Post subject: Re: cristobalite vs chalcedony in thin section Posted: Apr 08, 2025 21:34 Post subject: Re: cristobalite vs chalcedony in thin section |
|
|
| Thank you, gentlemen, for this interesting discussion. I observed very similar looking millimeter-size spherules in gypsum in Archidona, Spain: https://www.mindat.org/loc-257672.html - and I had been wondering how they formed.
|
|
| Back to top |
|
 |
marco campos-venuti

Joined: 09 Apr 2014
Posts: 240
Location: Sevilla



|
 Posted: Apr 09, 2025 06:32 Post subject: Re: cristobalite vs chalcedony in thin section Posted: Apr 09, 2025 06:32 Post subject: Re: cristobalite vs chalcedony in thin section |
|
|
Hi Alfredo,
perhaps you are referring to the gypsum of Mina Rica, Pulpì, Almeria, Spain, where gypsum crystals show inclusions of celestine needles and white spherulites. In my opinion, in that case they are bacterial colonies. I have found similar ones in some quartz (see my book "Biominerals"). Dissolution spherulites are common in glass (pitting) and in some microcrystalline silica, but I don't know if they are possible in crystalline material.
|
|
| Back to top |
|
 |
Daniel Garcia
Joined: 02 May 2022
Posts: 23
Location: Narbonne


|
 Posted: Apr 09, 2025 11:50 Post subject: Re: cristobalite vs chalcedony in thin section Posted: Apr 09, 2025 11:50 Post subject: Re: cristobalite vs chalcedony in thin section |
|
|
Thanks everybody for feedback and suggestions.
Let me add some more pictures of chalcedony flowers growing inside gypsum cristals from the same evaporite formation.
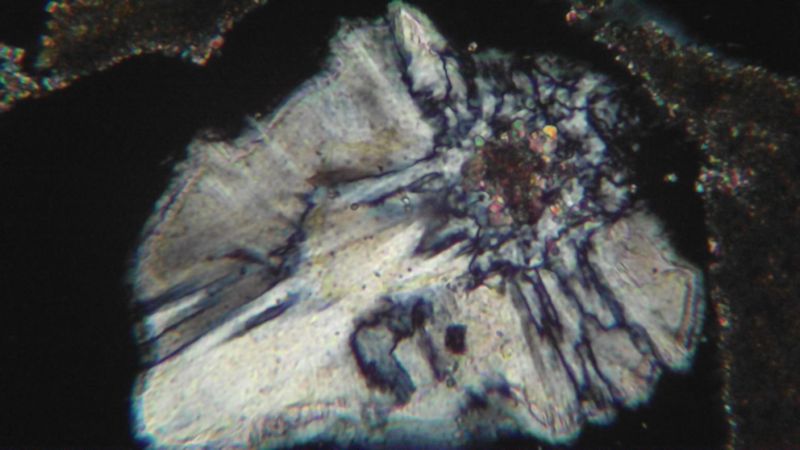
Incidentally, Marco, it seems that growth from a face is not the most common case ; very often, a carbonate impurity, a wood fragment or another undetermined inclusion in the gypsum may serve as a nucleation center for the chalcedony flower.
| Mineral: | chalcedony |
| Dimensions: | 1.5 x 2 mm |
| Description: |
XPL chalcedony flower growing in gypsum.
Chalcedony is in contact with native sulfur on the left and growth against gypsum on the right |
|
| Viewed: |
21017 Time(s) |

|
| Mineral: | chalcedony |
| Dimensions: | 1.5 x 2 mm |
| Description: |
| PPL view, of chalcedony growing in gypsum, native sulfur on the left |
|
| Viewed: |
21017 Time(s) |

|
| Mineral: | chalcedony |
| Dimensions: | 1.5 x 2 mm |
| Description: |
| XPL view, chalcedony in gypsum |
|
| Viewed: |
21013 Time(s) |

|
| Mineral: | chalcedony |
| Dimensions: | 1.5 x 2 mm |
| Description: |
| PPL view, chalcedony in gypsum |
|
| Viewed: |
21025 Time(s) |

|
| Mineral: | chalcedony |
| Dimensions: | 0.5 x 0.5 mm |
| Description: |
| XPL view ; note carbonate or clay impurity in the core of the chalcedony |
|
| Viewed: |
21013 Time(s) |
|
| Mineral: | chalcedony |
| Dimensions: | 0.5 x 0.5 mm |
| Description: |
|
| Viewed: |
21016 Time(s) |
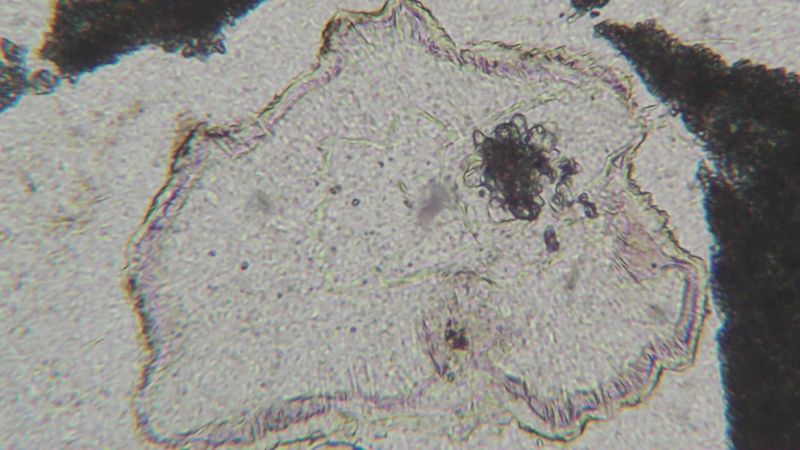
|
|
|
| Back to top |
|
 |
|





