| View previous topic :: View next topic |
| Author |
Message |
Gerhard Niklasch
Joined: 27 Mar 2009
Posts: 134
Location: Munich



|
 Posted: Feb 25, 2014 02:49 Post subject: Re: Daylight Fluorescent Hyalite Opal from Mexico Posted: Feb 25, 2014 02:49 Post subject: Re: Daylight Fluorescent Hyalite Opal from Mexico |
|
|
Thank you much for the clarification - yes, now the point is clear! It would have been something else if a 50µg sample had rivaled 60µg of Autunite. ;)
I'll soon have a chance to look at the luminescent response through my spectroscope, and will report on what I see. Unfortunately I have no way of capturing this photographically.
Cheers, Gerhard
|
|
| Back to top |
|
 |
Gerhard Niklasch
Joined: 27 Mar 2009
Posts: 134
Location: Munich



|
 Posted: Mar 01, 2014 15:17 Post subject: Re: Daylight Fluorescent Hyalite Opal from Mexico Posted: Mar 01, 2014 15:17 Post subject: Re: Daylight Fluorescent Hyalite Opal from Mexico |
|
|
Pleased to report that a specimen was delivered to my door today, and was eagerly unwrapped! Even behind closed windows under completely overcast skies, it is all yellowish-green and greenish-yellow (in separate intercalating areas) contrasting nicely with the sandstone(?) matrix. A white LED lamp strengthens the effect. It turns bright green under LWUV, and brighter yet under SWUV (I needn't even bother to darken the room!), showing (as expected) the marked uranyl bands when viewed through the spectroscope. While either UV wavelength is capable of exciting all the bands up to the blue-green one, it seems the blue component of the cloud and glass filtered daylight mostly excites the two or three bands towards the lower-energy, yellow side of the spectrum, resulting in a yellower hue. (The variation in hue across the specimen area under daylight or LED light might be due to varying concentrations of some co-activator.)
Excitedly (me too! :) ),
Gerhard
|
|
| Back to top |
|
 |
Mark Ost

Joined: 18 Mar 2013
Posts: 516
Location: Virginia Beach



|
 Posted: Mar 01, 2014 18:28 Post subject: Re: Daylight Fluorescent Hyalite Opal from Mexico Posted: Mar 01, 2014 18:28 Post subject: Re: Daylight Fluorescent Hyalite Opal from Mexico |
|
|
| Look forward to getting mine. Now if all our fingers fall off due to the Uranium content..........................
|
|
| Back to top |
|
 |
Gerhard Niklasch
Joined: 27 Mar 2009
Posts: 134
Location: Munich



|
 Posted: Mar 02, 2014 15:45 Post subject: Re: Daylight Fluorescent Hyalite Opal from Mexico Posted: Mar 02, 2014 15:45 Post subject: Re: Daylight Fluorescent Hyalite Opal from Mexico |
|
|
Well, for a typical hand specimen of this material, we're talking about an order of magnitude of one U238 decay per second on average, plus the same rate from each of the unstable daughter isotopes along the decay chain (that's what "secular equilibrium" amounts to). This really is low activity. If I were to worry about my fingers, I'm more worried of cutting myself on a cooking utensil or pinching them in a door by sheer personal stupidity. :)
I've played around a bit more with my LED flashlight and found that the yellow and green patches move when I move the light source: so they don't arise from different material properties at all. Rather, the opal blobs act as little lenses, focusing the incoming light onto a few bright spots on the rear side where they contact the matrix - and these spots move as the angle of illumination changes. And then the blobs again act as loupes, showing magnified versions of the bright spots to the observer's eyes. They now appear yellow because the violet component of the incoming light has already been absorbed and turned into green fluorescence. Those bits of opal through which I'm not looking at such a bright spot appear green rather than yellow.
Along the way, I also learned that this particular LED flashlight emits practically no blue light… it contrives somehow to produce a continuous spectrum from red through yellow to green, then there's a gap, and then there's a violet band centered around 450-460nm; the whole arranged to fool a human eye into seeing white when there's no spectroscope in the light path.
Cheers, Gerhard
|
|
| Back to top |
|
 |
alfredo
Site Admin

Joined: 30 Jan 2008
Posts: 1013



|
 Posted: Mar 02, 2014 16:20 Post subject: Re: Daylight Fluorescent Hyalite Opal from Mexico Posted: Mar 02, 2014 16:20 Post subject: Re: Daylight Fluorescent Hyalite Opal from Mexico |
|
|
| Azurite must look awful in that light ;((
|
|
| Back to top |
|
 |
Mark Ost

Joined: 18 Mar 2013
Posts: 516
Location: Virginia Beach



|
 Posted: Mar 02, 2014 17:41 Post subject: Re: Daylight Fluorescent Hyalite Opal from Mexico Posted: Mar 02, 2014 17:41 Post subject: Re: Daylight Fluorescent Hyalite Opal from Mexico |
|
|
| Ok, so maybe it's not our fingers but you never know about low level radiation! Jordi may have to support my five eyed children.
|
|
| Back to top |
|
 |
Mark Ost

Joined: 18 Mar 2013
Posts: 516
Location: Virginia Beach



|
 Posted: Mar 02, 2014 17:53 Post subject: Re: Daylight Fluorescent Hyalite Opal from Mexico Posted: Mar 02, 2014 17:53 Post subject: Re: Daylight Fluorescent Hyalite Opal from Mexico |
|
|
| Now that you mention it Pierre, it occurred to me I had a beryl from the same location with opal and it seems to do the same thing yours does. Much more subtle than the Mexican opal but it too reacts in daylight.
|
|
| Back to top |
|
 |
Mark Ost

Joined: 18 Mar 2013
Posts: 516
Location: Virginia Beach



|
 Posted: Mar 02, 2014 18:03 Post subject: Re: Daylight Fluorescent Hyalite Opal from Mexico Posted: Mar 02, 2014 18:03 Post subject: Re: Daylight Fluorescent Hyalite Opal from Mexico |
|
|
Pete
I just checked my Namibian beryl (aquamarine) / schorl/ opal. Under incandescent light it appears a frosted white with no hint of green color. In the late evening light it takes on a green hue. Glass does not seem to filter the effect. I will try in direct sunlight tomorrow. Fluorescent light seems to wash it out. Standing away from a south facing window the green is evident but washes out closer to the window due to brightness. Very responsive to SW, MW even in daylight. Less so LW.
|
|
| Back to top |
|
 |
alfredo
Site Admin

Joined: 30 Jan 2008
Posts: 1013



|
 Posted: Mar 02, 2014 18:38 Post subject: Re: Daylight Fluorescent Hyalite Opal from Mexico Posted: Mar 02, 2014 18:38 Post subject: Re: Daylight Fluorescent Hyalite Opal from Mexico |
|
|
| I tried to measure the radioactivity of daylight fluorescent opal with a geiger counter and got nothing above background. Ditto for highly fluorescent artificial "uranium glass". The amount of uranium present is simply too low. As for the dangers of low-level radiation, remember that we are surrounded by it all the time; a specimen of daylight fluorescent uranyl-bearing hyalite seems to add no appreciable quantity to that level. Nothing to worry about.
|
|
| Back to top |
|
 |
Cesar M. Salvan
Site Admin
Joined: 09 Jun 2008
Posts: 127
Location: Alcalá de Henares



|
 Posted: Mar 02, 2014 19:01 Post subject: Re: Daylight Fluorescent Hyalite Opal from Mexico Posted: Mar 02, 2014 19:01 Post subject: Re: Daylight Fluorescent Hyalite Opal from Mexico |
|
|
| As I said in my report, the uranium content can be detected only by sensitive instruments. Hand-held Geiger monitors are not adequate to detect it. GM monitors equipped with thin window detectors, used correctly, can detect the small amount of activity of these samples, as are too close to background.
|
|
| Back to top |
|
 |
Jesse Fisher

Joined: 18 Mar 2009
Posts: 639
Location: San Francisco



|
 Posted: Mar 02, 2014 23:17 Post subject: Re: Daylight Fluorescent Hyalite Opal from Mexico Posted: Mar 02, 2014 23:17 Post subject: Re: Daylight Fluorescent Hyalite Opal from Mexico |
|
|
| At the TGMS show I measured the activity level of a specimen using a hand-held scintallometer that was available in the GSA booth in the convention center lobby. The measured activity level was 1-3 microSieverts per hour and 0. - 0.3 milliRoentgens per hour. According to references, the former measurement is about the some as the exposure one will receive while flying in a typical airliner at cruising altitude (around 40,000 feet). I'm not sure how many hours I have logged flying at 40,000 feet in the many trips between California and London over the past 20 years, but I am sure it is far more than I would ever spend holding one of these specimens, no matter how pretty they are!
|
|
| Back to top |
|
 |
Cesar M. Salvan
Site Admin
Joined: 09 Jun 2008
Posts: 127
Location: Alcalá de Henares



|
 Posted: Mar 09, 2014 16:11 Post subject: Re: Daylight Fluorescent Hyalite Opal from Mexico Posted: Mar 09, 2014 16:11 Post subject: Re: Daylight Fluorescent Hyalite Opal from Mexico |
|
|
I received a lot of questions by email and even by phone, about this opal and its uranium content. Two of these questions are 'faq', so I decided to share it here for general information:
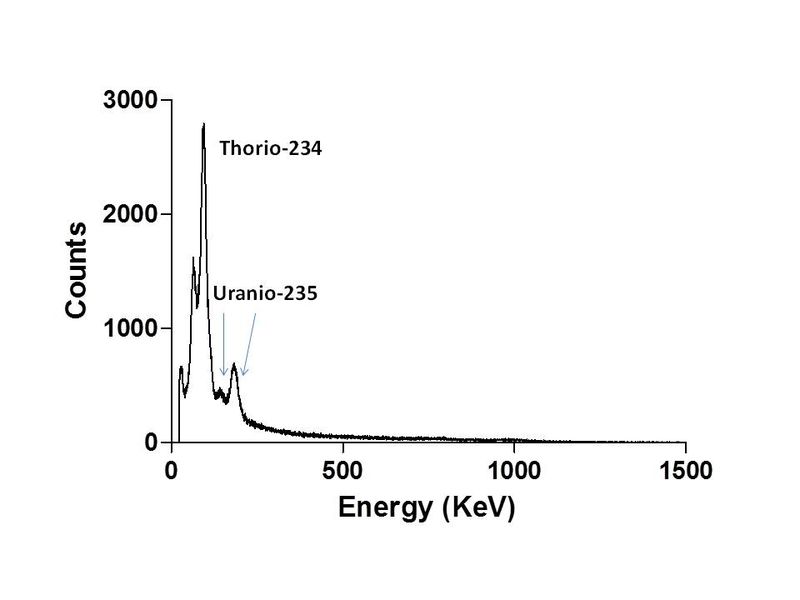
- In the spectrum of the radioactivity of the opal, why appears uranium-235 and other elements? if U-238 is the major isotope in natural uranium, where is it?. The U-235 is dangerous?
There is certain concern about uranium. Some people does not expect the presence of U-235, well known by their nuclear uses. Well, the gamma spectrum showed in my report is the typical radioactiviy spectrum of uranium in naturally occurring radioactive materials (NORM), as rocks or minerals. If we measure the spectrum of U in any mineral or rock, is always the same.
The main component in uranium, the U-238 (99.28% of natural uranium) is an element with a long half-life and, hence, a poor radioactive emitter. It is transmutated by alpha emission in Thorium-234. This transformation is accompanied by emission of gamma rays, but with a very low energy. Its energy is predated in the spectrum by the daughter nuclides and the X-ray emission. This is why, despite its abundance, the U-238 is invisible in this spectrum. The U-235 has a lower half-life and is a more intense emitter. The majority of radiation emitted natural uranium does not come from uranium, but from its daughter nuclides in the uranium series. These nuclides appears at constant concentration in NORM, because the uranium older than 700000 years is in "secular equilibrium". This is why the spectrum is the same in all minerals and rocks and this is why the uranium in minerals is more radioactive than purified uranium.
Do not be afraid by terms as uranium, X-ray, etc. This is a completely natural process and, in the quantities contained in opals, is completely safe.
- Is possible that the opals are a fake, produced by treatment of opals with uranium salts?
This is absolutely impossible. And, in the hypothetical case of a fake, the gamma spectrum would reveal the fraud, as the spectrum of uranium in laboratory chemicals or other processed materials is completely different.
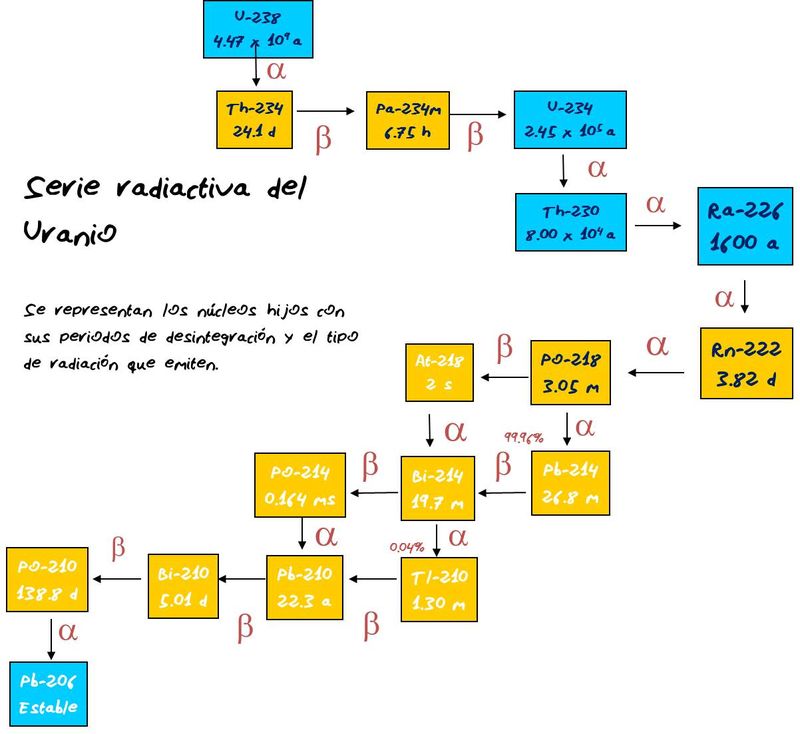
| Description: |
| The uranium decay series, which finish in the "radiogenic lead". The proportion between U and radiogenic lead is very useful in geologic dating |
|
| Viewed: |
28499 Time(s) |
|
| Description: |
| Spectrum of natural uranium after resetting (i.e., purification from other components) and waiting one year to gain equilibrium with its first daughter in the series. Note the peak of the 235 isotope. This isotope is purified for use in nuclear fuel and the residue is the "depleted uranium". |
|
| Viewed: |
28414 Time(s) |
|
|
|
| Back to top |
|
 |
Peter Megaw
Site Admin

Joined: 13 Jan 2007
Posts: 973
Location: Tucson, Arizona



|
 Posted: Mar 18, 2014 14:12 Post subject: Re: Daylight Fluorescent Hyalite Opal from Mexico Posted: Mar 18, 2014 14:12 Post subject: Re: Daylight Fluorescent Hyalite Opal from Mexico |
|
|
I have been playing around with another piece of this material and have taken a series of new pictures showing the different fluorescent responses to various lighting conditions. (Incandescent W light; daylight; a combination of Incandescent and SWUV and full SWUV).
I discovered that there appears to be a distinct difference in the daylight fluorescence between early morning (8:00) versus early afternoon (14:00); the later is stronger and yellower than the early shot. Both were taken in the same place under full shade with otherwise unrestricted view to the sun. No cloud cover whatsoever. The only real difference in the photographic conditions seems to have been the sun's position...and of course amount of atmospheric filtering.
All the photographs are of the same piece
| Description: |
Hyalite Opal
Central Mexico
170 x 100 mm
Hyalite opal photographed under 100 watt W incandescent light |
|
| Viewed: |
28277 Time(s) |

|
| Description: |
Hyalite Opal
Central Mexico
170 x 100 mm
Hyalite opal photographed under full shade at 8 am |
|
| Viewed: |
28268 Time(s) |

|
| Description: |
Hyalite opal
Central Mexico
170 x 100 mm
Hyalite opal photographed under full shade at 14:00 in exactly the same place as 8 am shot |
|
| Viewed: |
28288 Time(s) |

|
| Description: |
Hyalite Opal
Central Mexico
170 x 100 mm
Hyalite opal photographed with a combination of W Incandescent light and SWUV |
|
| Viewed: |
28265 Time(s) |

|
| Description: |
Hyalite Opal
Central Mexico
170 x 100 mm
Hyalite Opal photographed under SWUV |
|
| Viewed: |
28250 Time(s) |

|
_________________
Siempre Adelante! |
|
| Back to top |
|
 |
Vinoterapia
Joined: 03 Feb 2009
Posts: 181
Location: Houston, Tx



|
 Posted: Mar 19, 2014 08:59 Post subject: Re: Daylight Fluorescent Hyalite Opal from Mexico Posted: Mar 19, 2014 08:59 Post subject: Re: Daylight Fluorescent Hyalite Opal from Mexico |
|
|
| Amazing, this really is a kind of "Chameleon" mineral.
|
|
| Back to top |
|
 |
waterdog
Joined: 31 Dec 2012
Posts: 10


|
 Posted: Mar 24, 2014 22:19 Post subject: Re: Daylight Fluorescent Hyalite Opal from Mexico Posted: Mar 24, 2014 22:19 Post subject: Re: Daylight Fluorescent Hyalite Opal from Mexico |
|
|
Our specimen arrived today, thanks Jordi!
I checked the specimen with a Ludlum Type 3 radiation detector with a Model 44-9 pancake probe. The probe picks up alpha/beta/gamma. The response was 600 counts per minute at 0.5 cm from the detector face. The sample covered about 70% of the detector face. This count translates to 0.2 mrem/hour (2 μSv/hour), a worthless number for actual dose calculations but it gives a crude estimate of the radioactivity of the sample. The sample clearly radiates above background (less than 50 counts per minute in a dusty room). The specimen kicks out 18 mSv/yr while the average annual radiation dose (in US) is 6.2 mSv/year. So don't keep one in your pocket. :)
P.S. The meter hasn't been calibrated in 20 years.
|
|
| Back to top |
|
 |
|




