| View previous topic :: View next topic |
| Author |
Message |
silvia
Joined: 10 Oct 2021
Posts: 269
Location: UK



|
 Posted: Aug 18, 2022 16:20 Post subject: Re: Pseudomorph chemistry - (40) Posted: Aug 18, 2022 16:20 Post subject: Re: Pseudomorph chemistry - (40) |
|
|
| James Catmur wrote: | | One I collected back in 1983, from land being cleared so not a mine |
Nice solid specimen of Goethite. We often hear the comment about minerals being natural art, and perhaps that is true, but I am inclined to think it is just a marketing ruse, what is often overlooked is the true beauty and that beauty lies in the way atoms arrange themselves to form minerals. My partner and I find beauty not only in well crystallized mineral specimens but also massive specimens that to some are just common ore samples.
Again nice piece – truly!
|
|
| Back to top |
|
 |
James Catmur
Site Admin

Joined: 14 Sep 2006
Posts: 1489
Location: Cambridge



|
 Posted: Aug 19, 2022 05:59 Post subject: Re: Pseudomorph chemistry - (40) Posted: Aug 19, 2022 05:59 Post subject: Re: Pseudomorph chemistry - (40) |
|
|
It is the cast it made of the long-lost fluorite that I love - they were clearing land near the road so I had to stop and see what was turning up.
| silvia wrote: | | Nice solid specimen of Goethite. |
|
|
| Back to top |
|
 |
Daniel Garcia
Joined: 02 May 2022
Posts: 23
Location: Narbonne


|
 Posted: Aug 20, 2022 11:11 Post subject: Re: Pseudomorph chemistry - (40) Posted: Aug 20, 2022 11:11 Post subject: Re: Pseudomorph chemistry - (40) |
|
|
| Roger Warin wrote: | Hello Daniel
I am obviously interested in additional information.
Can you give your opinion on the azurite – malachite transformation?
I assume that the phenomenon of pseudomorphosis is more common than we think.
Thanks. |
Hi Roger,
I read the Vink's paper, a good exercise of aqueous thermochemistry, perhaps a bit more complicated than strictly necessary, but still useful to delineate the stability relationships of azurite and malachite at room temperature.
I'm afraid the inference that can be made from this kind of data will be somewhat disappointing for people interested in pseudomorphism. Thermodynamics can tell you how much reaction can (or must) take place under given conditions, how much secondary minerals you may form, but very little about the morphological appearance of the reaction products: no thermodynamic model can predict whether the secondary mineral (malachite) will precipitate by replacing a primary one (azurite) or simply by precipitating in the remaining voids somewhere else.
This is because thermodynamics is a macroscopic science, while people in this forum are expecting answers (about pseudomorphism) at the grain scale, where the reaction mechanisms and kinetics (at the microscopic level) matters.
|
|
| Back to top |
|
 |
Roger Warin

Joined: 23 Jan 2013
Posts: 1250



|
 Posted: Aug 20, 2022 23:17 Post subject: Re: Pseudomorph chemistry - (40) Posted: Aug 20, 2022 23:17 Post subject: Re: Pseudomorph chemistry - (40) |
|
|
Hello Daniel,
Thank you very much for your nuanced answer.
Away from laboratories for a long time (which gives me the opportunity to reflect on the transformations of minerals and even their origin (since I love to interpret thin sections of meteorites) without future risks for my career!
I still meet professors of mineralogy or geochemistry, when I ask questions of this kind, only a vague idea is given, and often it is the thought of a guru which takes precedence. This has been written and recorded in scientific articles.
The advantage of age is that we no longer fear the wrath of the defenders of exact science.
To consider only the (irreversible) azurite-malachite transition, I only have my (optical) microscopes. Azurite is a sort of metastable chemical state that may or may not break depending on the fortuitous arrival of external agents, as anions.
As a collector of Tsumeb minerals, I have seen perfect azurites and other specimens completely transformed into malachite.
These are obviously not perimorphoses.
The perfect azurite thus undergoes chemical degradation.
In a 1st stage, I thought of a diffusion of atoms, but these processes are extremely slow (seen in meteorites).
For the azurite-malachite pseudomorphosis, it is indeed a chemical degradation (anionic) of probable origin of hydrothermal nature. It seems to me that we have to accept a temperature-pressure anomaly.
This transformation takes place in situ, in the host crystal: the copper remains in place and the anions are exchanged and replaced.
As only proof, I have only what I see. On fine specimens, it can be seen that the fine textures of the crystals (like streaks) of azurite can be preserved.
We are very far from the “pseudomorphosis of a nail into a totally rusty nail”. This trivial example shows the importance of the nature of the new molecule. Also, rust is an ill-defined mineral occupying a larger volume than the nail. At the limit, the initial shape of the nail disappears.
The volume occupied by the replacement mineral therefore affects this metamorphosis.
If the morphology of the azurite crystal is preserved, it is because the malachite can remain confined in the host crystal in the first approximation.
Here is my first thought. I can abandon it in favor of another scenario.
|
|
| Back to top |
|
 |
Daniel Garcia
Joined: 02 May 2022
Posts: 23
Location: Narbonne


|
 Posted: Aug 28, 2022 05:45 Post subject: Re: Pseudomorph chemistry - (40) Posted: Aug 28, 2022 05:45 Post subject: Re: Pseudomorph chemistry - (40) |
|
|
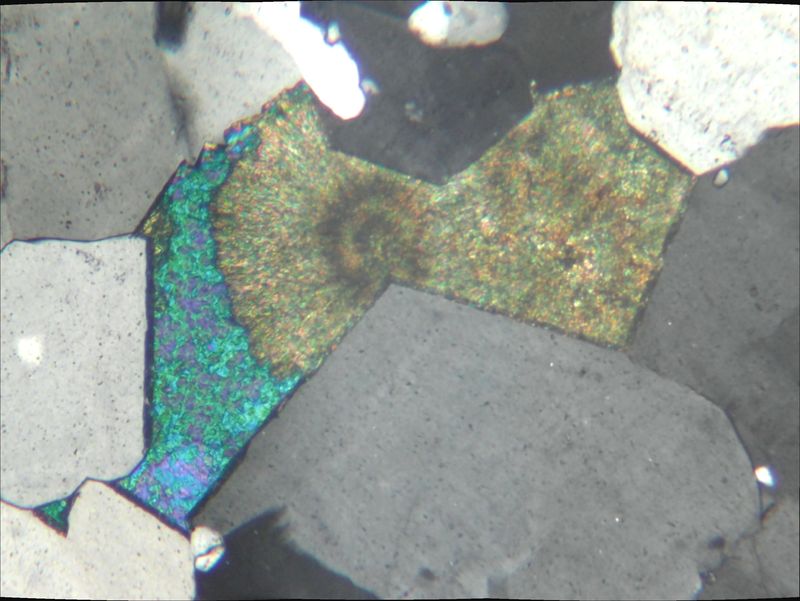
Here is a thin section of a porous rock (mostly quartz) with pore filling azurite (blue) being partly converted to malachite (fibers). Looks similar to some of the Tsumeb specimens previously posted on this forum.
| Mineral: | azurite |
| Dimensions: | 0.5 mm |
| Description: |
| azurite partly converted to malachite |
|
| Viewed: |
36663 Time(s) |
|
|
|
| Back to top |
|
 |
Roger Warin

Joined: 23 Jan 2013
Posts: 1250



|
 Posted: Aug 28, 2022 09:28 Post subject: Re: Pseudomorph chemistry - (40) Posted: Aug 28, 2022 09:28 Post subject: Re: Pseudomorph chemistry - (40) |
|
|
Thank you Daniel for this informative photo.
I presume the observation was made in polarized light.
What would cross-polarized vision look like?
Can I say that the area on the left is an azurite crystal already partially transformed into malachite?
You are very lucky to have TS of different rocks.
I will soon give my macroscopic photos which take into account the external morphology.
|
|
| Back to top |
|
 |
Daniel Garcia
Joined: 02 May 2022
Posts: 23
Location: Narbonne


|
 Posted: Aug 28, 2022 13:43 Post subject: Re: Pseudomorph chemistry - (40) Posted: Aug 28, 2022 13:43 Post subject: Re: Pseudomorph chemistry - (40) |
|
|
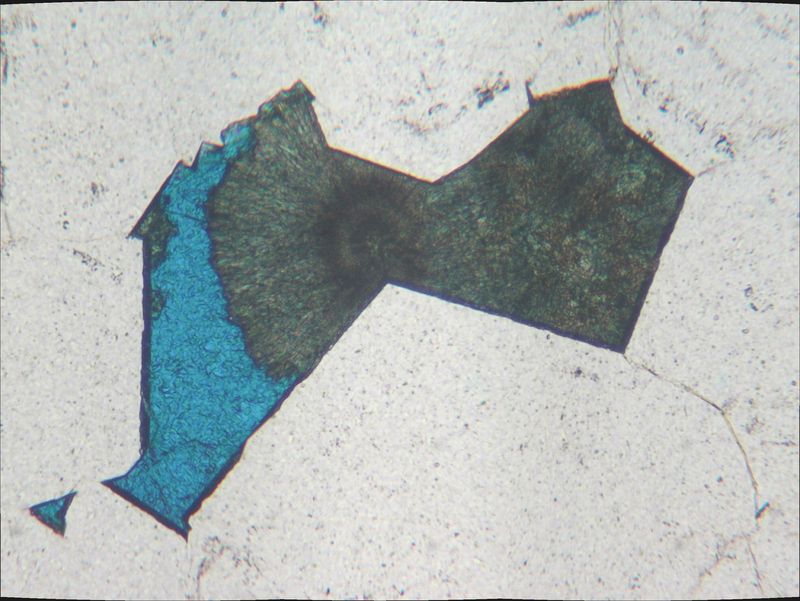
Thanks Roger
Here is the same view under normal light.
Azurite is still blue. It seems to be one single crystal, that inherits its shape from the pore geometry. Some other pores in the same section are filled with azurite only, and some others with boitroidal malachite. This one is the only one where the replacement is "ongoing"
| Mineral: | azurite |
| Dimensions: | 0.5 mm |
| Description: |
|
| Viewed: |
19463 Time(s) |
|
|
|
| Back to top |
|
 |
Roger Warin

Joined: 23 Jan 2013
Posts: 1250



|
 Posted: Aug 29, 2022 04:19 Post subject: Re: Pseudomorph chemistry - (40) Posted: Aug 29, 2022 04:19 Post subject: Re: Pseudomorph chemistry - (40) |
|
|
Hi Daniel and all lovers of pseudomorphs,
Still about my malachite pseudomorph after azurite.
A general macrophoto shows the appearance of this specimen.
This old name corresponds well to the destructive sequence of azurite in malachite. The time scale is long under normal conditions.
This phenomenon corresponds to a natural alteration of the initial azurite (unit cell vol.= 301.97 A³) into malachite (unit cell vol.= 364.35 A³).
It was taught to me in the 1950s (and certainly before) but without a description of the chemical process.
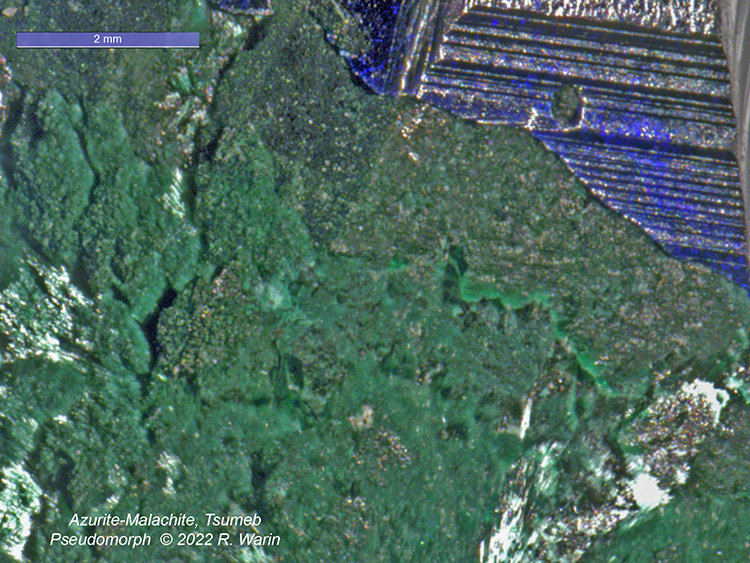
To highlight this process, I have chosen a strange little sample of Tsumeb, although it takes faith to observe it!
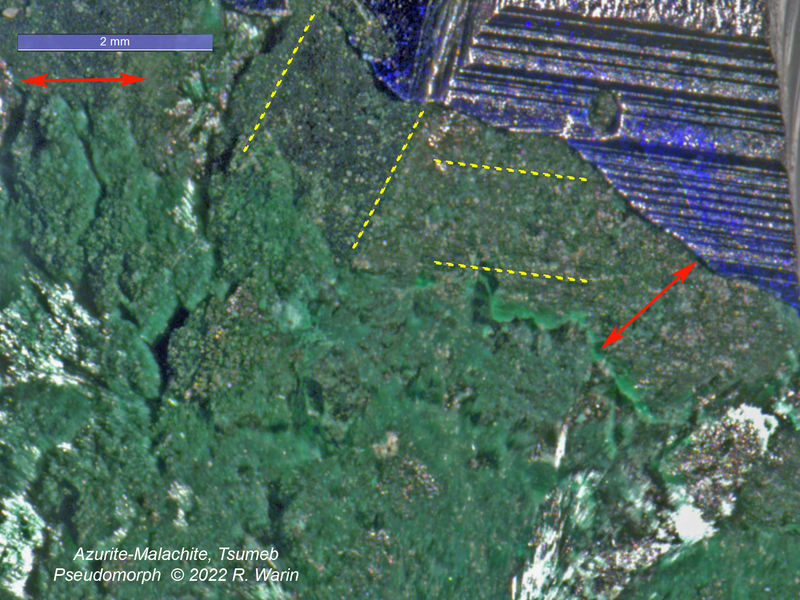
Azurite-malachite#1-02-A_RIn this view (FOV=8 mm), I have the impression of seeing the initial shape of the azurite crystal continue downwards, with however a gradual increase in volume.
Even in the region of the dotted lines, one notes (it seems to me) a certain differentiation in the granulation of the grains of malachite, over an elongation of approximately 2 mm at the most.
I remain convinced that this pseudomorphosis is the result of an intimate transformation of the initial mineral, without the presence in the solid state of liquid as such, but perhaps of coordinated water molecules. This is not a solution. The transition takes place, inside the crystal, even if isolated water molecules can act as ligands for the copper cations. However, the azurite crystal has been found in hydrothermal situations.
In a museum, partially azurite remains stable, in the Tsumeb mine not, depending on the circumstances.
I don't know if you can compare this process to the progressive rusting of a nail.
Then in a second time, this association served as the seed for a second generation of fibrous malachite under usual conditions.
This habitus of secondary malachite could perhaps specify the physicochemical conditions of the transition. What is odd is that this "dry rot" did not attack the rest of the azurite crystal.
| Mineral: | Azurite-malachite psm |
| Locality: | | Tsumeb Mine, Tsumeb, Otjikoto Region, Namibia |  |
|
| Dimensions: | c cm height |
| Description: |
|
| Viewed: |
19410 Time(s) |

|
| Description: |
|
| Viewed: |
19402 Time(s) |
|
| Description: |
|
| Viewed: |
19429 Time(s) |
|
|
|
| Back to top |
|
 |
Daniel Garcia
Joined: 02 May 2022
Posts: 23
Location: Narbonne


|
 Posted: Aug 29, 2022 08:15 Post subject: Re: Pseudomorph chemistry - (40) Posted: Aug 29, 2022 08:15 Post subject: Re: Pseudomorph chemistry - (40) |
|
|
Thanks Roger, for this nice "macro" case.
It seems to me that in the dotted area of your last picture, the termination of the malachite cristals simply mimic the striated outer shape of the former azurite.
|
|
| Back to top |
|
 |
Cesar M. Salvan
Site Admin
Joined: 09 Jun 2008
Posts: 127
Location: Alcalá de Henares



|
 Posted: Aug 30, 2022 12:22 Post subject: Re: Pseudomorph chemistry - (40) Posted: Aug 30, 2022 12:22 Post subject: Re: Pseudomorph chemistry - (40) |
|
|
| RayStraw wrote: | I have a 11cm high broken azurite which shows internal growth of botryoidal malachite.
The rough chemistry requires 2 portions of solid azurite and one portion of liquid water to yield three portions of solid malachite and one portion of carbon dioxide (gas?). (The reaction yields 9% porosity as malachite is more dense than azurite.)
Is there an intermediate reaction that dissolves the azurite to permit in the formation of malachite? I assume the reaction is at the sub microscopic level.
Any comments would be appreciated including the pH of the dissolution. |
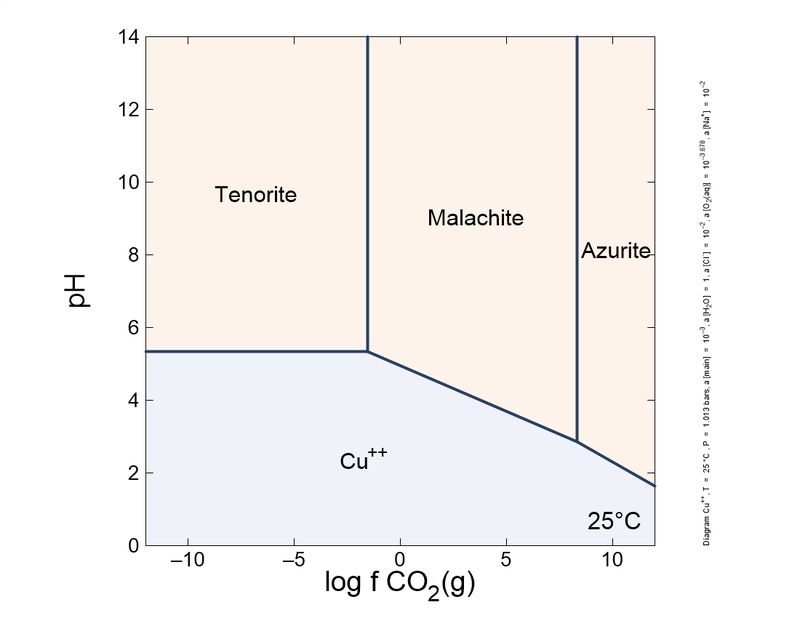
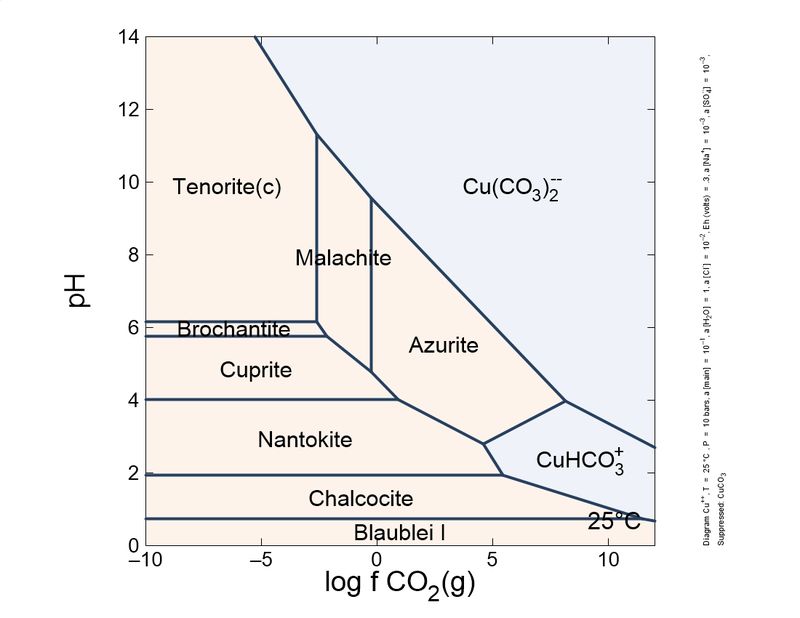
Hi, This is my (very simplified) take on this question. I draw the species stability in a pH/CO2 fugacity diagram. As you can see, malachite is the stable species at moderate CO2 pressure. The formation and stability of azurite or malachite depends on CO2 more than pH or water. Even the transformation takes place without contact with water. Azurite is 'richer' in carbon than malachite. In practical terms, to form and stabilize azurite in the lab, you requires a high pressure reactor with 40-50 bar CO2, and to keep azurite stable is necessary a CO2 fugacity of around 1 bar. Hence, all azurite slowly transforms into malachite, a process accelerated by temperature. This process is a problem in art conservation, where azurite pigment turned green with time in old paintings. So, probably, in 300 years almost all azurite crystals in collections will lose some blue color and turn green, in a reaction that involves the loss of CO2. Actually, the mechanistic details of the transformation are not well understood.
In standard conditions, the most stable phase is tenorite. But, malachite is the species towards other phases tends in supergenic alteration and it is stable enough. Malachite 'blackening' to tenorite have been observed, though, for example in malachite used as pigment.
The transformation of azurite into malachite involves a change in molar volume, from 91 cm3/mol of azurite to 55 cm3/mol of malachite. This contraction is a smoking gun of a pseudomorphosis; the pseudomorphized azurite crystal shows frequently cavities and deformations resulting from the contraction. This is helpful to distinguish pseudomorphs from redissolution-reprecipitation or sequential deposition of the carbonates, as it is also common to observe malachite covering azurite due to the reduction of CO2 fugacity during mineral formation.
| Description: |
|
| Viewed: |
19348 Time(s) |
|
|
|
| Back to top |
|
 |
Roger Warin

Joined: 23 Jan 2013
Posts: 1250



|
 Posted: Aug 30, 2022 13:48 Post subject: Re: Pseudomorph chemistry Posted: Aug 30, 2022 13:48 Post subject: Re: Pseudomorph chemistry |
|
|
| Really thank you for this demonstration.
|
|
| Back to top |
|
 |
Cesar M. Salvan
Site Admin
Joined: 09 Jun 2008
Posts: 127
Location: Alcalá de Henares



|
 Posted: Aug 30, 2022 18:43 Post subject: Re: Pseudomorph chemistry - (40) Posted: Aug 30, 2022 18:43 Post subject: Re: Pseudomorph chemistry - (40) |
|
|
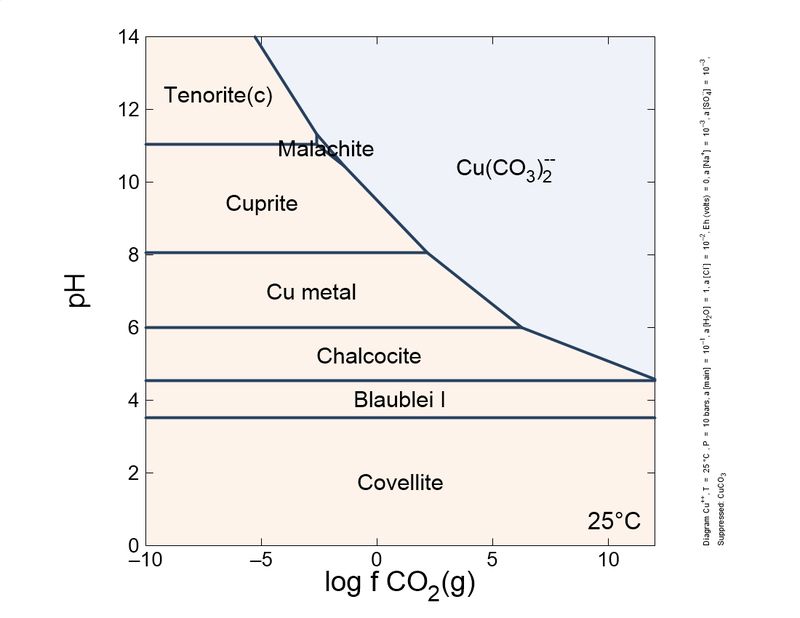
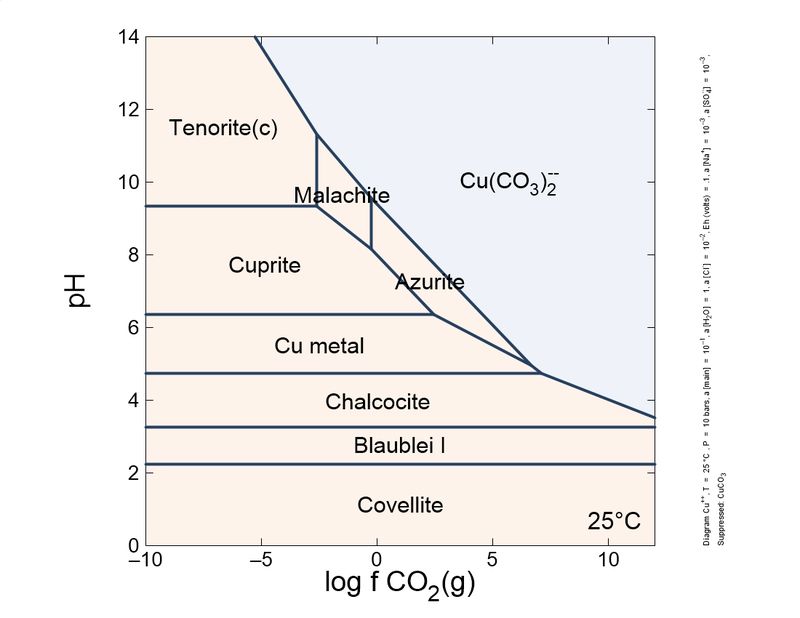
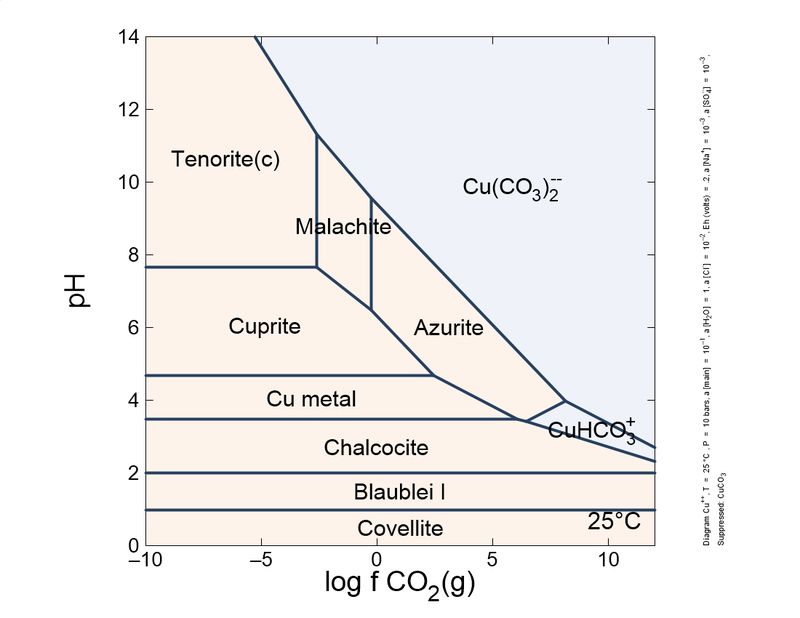
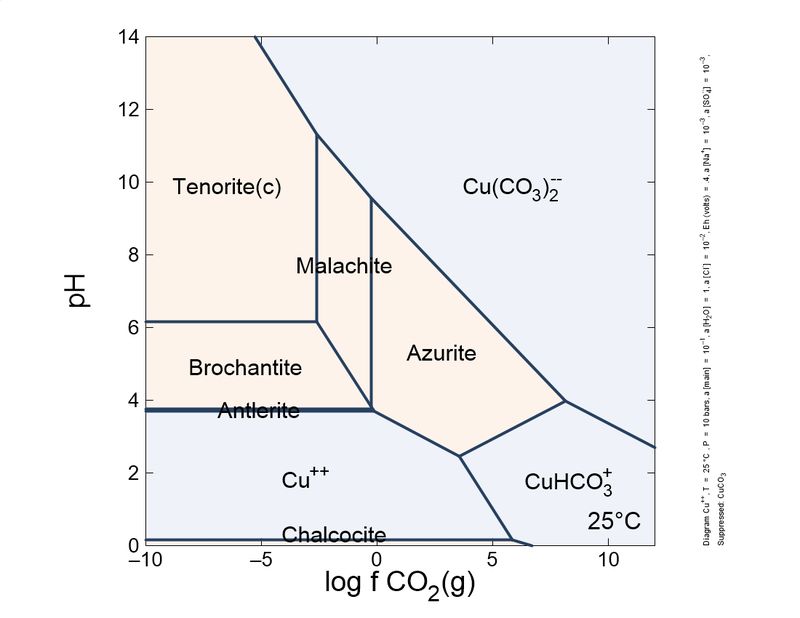
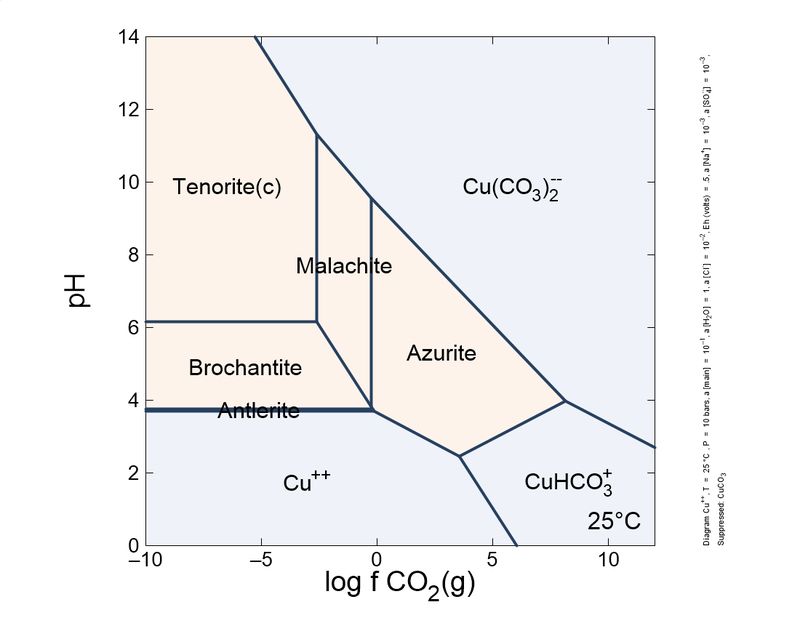
Following this little game. I changed slightly the calculations: I introduced a different, more actualized set of thermodynamic data, and also introduced sulfur species and copper mobile complexes.
To show it to you more practical and interesting, I prepared a sequence of plots, from reducing conditions to oxidant conditions. You can see the mineral sequence generated, which is very familiar to you all.
As you can see, the formation of carbonates is important and depends on the CO2 concentration. To keep azurite stable, a pure CO2 atmosphere is necessary. So, unless you store your azurite specimens in pure CO2, expect the slow transformation into malachite, which will fade the blue of your favorite azurites, giving it green tinges. This transformation is quite slow, but noticeable in a lifetime. Sunlight and heat increase the rate of the phase transition.
These schemes explain (very simplified) the most common copper mineral associations and sequences you find in your specimens. For example, it explains why brochantite is a common sulfate species, and antlerite is way less common. Also, to form antlerite a higher copper concentration is necessary. Overall, malachite occupies a central position and the most stable in the majority of conditions. This explains why it is very common.
"Cesar"-someone could ask-"in your plots, tenorite, and not malachite, is the thermodynamically favored species in normal atmosphere, whose log fCO2 is around -6. Why malachite does not turn black so easily as azurite turns green?". This could be explained by the additional stability of the malachite structure by Jahn-Teller effect, which is very important in copper coordination. To blacken the malachite more heat is necessary, to expel water and CO2, and break coordination octahedra.
| Description: |
| Eh 0 (slightly reducing conditions) |
|
| Viewed: |
19337 Time(s) |
|
| Description: |
| Eh 0.1 (very slightly oxidant) |
|
| Viewed: |
19326 Time(s) |
|
| Description: |
|
| Viewed: |
19298 Time(s) |
|
| Description: |
|
| Viewed: |
19305 Time(s) |
|
| Description: |
|
| Viewed: |
19287 Time(s) |
|
| Description: |
| Eh 0.5 (normal atmosphere) |
|
| Viewed: |
19314 Time(s) |
|
|
|
| Back to top |
|
 |
Daniel Garcia
Joined: 02 May 2022
Posts: 23
Location: Narbonne


|
 Posted: Aug 31, 2022 01:02 Post subject: Re: Pseudomorph chemistry - (40) Posted: Aug 31, 2022 01:02 Post subject: Re: Pseudomorph chemistry - (40) |
|
|
Hi everybody,
Thanks, Cesar, for this expanded picture of the stability relationships in the H2O O2 CO2 SO4 CuO system. I have two questions and a suggestion.
(1) Which is the thermodynamic database you used for these diagrams?
(2) Did somebody study the geometry of the replacement front between azurite and malachite in old pigments? Does it look like a corona as in common corrosion cases?
(3) and since the conversion of azurite to malachite at constant Cu involves the addition of water while removing some CO2, why not simply keeping our nice azurites in dried air to prevent greening...
|
|
| Back to top |
|
 |
RayStraw
Joined: 21 Jul 2018
Posts: 47
Location: Bloomington Illinois


|
 Posted: Sep 10, 2022 15:13 Post subject: Re: Pseudomorph chemistry - (40) Posted: Sep 10, 2022 15:13 Post subject: Re: Pseudomorph chemistry - (40) |
|
|
This is very interesting.
Please advise the locality.
Ray
|
|
| Back to top |
|
 |
|





